Borax Formula
Borax, also known as sodium tetraborate or sodium borate, is a mineral and inorganic salt largely used as ingredients in laundry and cleaning products.
Formula and structure: Borax can be found as anhydrous, pentahydrate or decahydrated salt. The anhydrous and decahydrate forms are the most common. The borax decahydrate chemical formula is Na2B4O7.10H2O (also written as Na2[B4O5(OH)4]·8H2O) and its molecular formula is H20B4Na2O17. The borax anhydrous molar mass is 201.22 g mol-1, while the decahydrate salt molar mass is 381.38 g mol-1. Borax decahydrate is a complex formed by the ion [B4O5(OH)4]-2, which has 4 boron atoms coordinated to 7 oxygen atoms and formed a bicyclic structure. The chemical structures for the anhydrous (planar and 3-D) and decahydrated salt can be written as below:
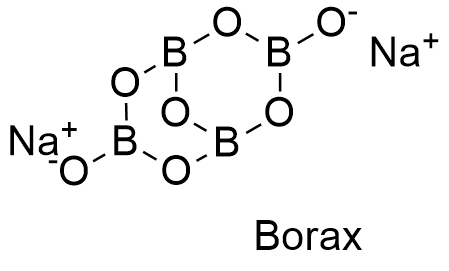
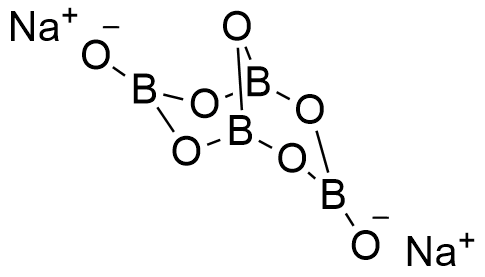
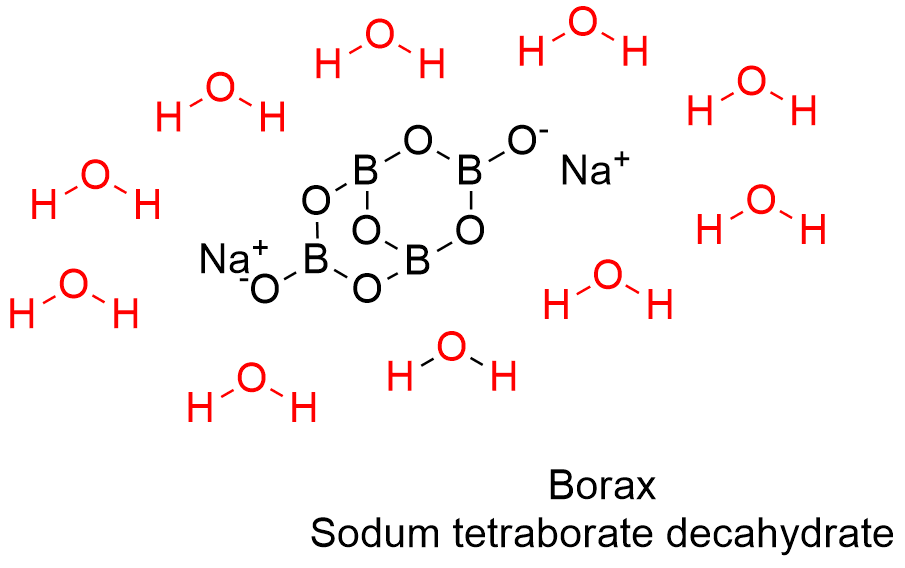
Occurrence: Borax can be found in dry lake deposits in California, USA and Turkey. Furthermore, those lakes also provide other borate minerals that can be treat to obtain borax.
Preparation: Borax is mostly extracted from natural source. Consequently, there are few industrial process to prepare it by synthetic routes. Other important source of borax are the borate minerals found at the same lakes that borax. Borax and related borate minerals are purified by recrystallization.
Physical properties: Borax is a white crystalline powder. Its melting and boiling points are 75 ºC and 320 ºC, respectively. Borax density is 1.73 g mL-1. It is highly soluble in water.
Chemical properties: Borax in water form alkaline solutions. In acid solution, borax reacts to produce other borates such as sodium perborate (PBS), a compound very used in chemical industry. It can also form boric acid:
Na2B4O7·10H2O + 2 HCl → 4 H3BO3 + 2 Na+ + 2 Cl- + 5 H2O
Uses: Borax is largely used by chemical, food and pharmaceutical industries. Borax is used as antifungal additive in medicine and food manufacture; it is also use in these industries to prepare buffer solution to regulate the pH of products. It is extensively used as an ingredients of many detergents, laundry and cleaning products. Borax is an alternative to mercury use in gold mining and it is also used as a neutron-capture in nuclear reactors.
Health effects/safety hazards: Borax is not toxic by ingestion and it is not irritating. However, some studies are being doing to show its possible carcinogenic effects and its risk over fertility and pregnancy. It is not flammable and it does not reacts with other chemical compounds.
|
Related Links: |
Related Topics
Boric acid Formula - Boric Acid Uses, Properties, Structure and ...
Hydrazine Formula - Hydrazine Uses, Properties, Structure and ...
Titration Formula
