Caffeine Formula
Caffeine, known by the IUPAC name 1, 3, 7-Trimethylpurine-2,6-dione, is an organic compound that are part of the list of the Most Essential Medicines of the WHO. It is also part of the much extended drink coffee and is a very popular stimulant.
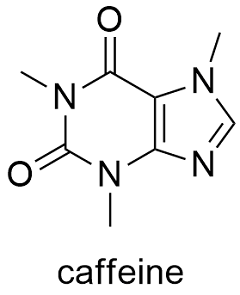
Formula and structure: The caffeine chemical formula is C8H10N4O2 and its molar mass is 194.19 g mol-1. The molecule is a typical natural alkaloid, formed by a pyrimidinedione (6-member ring with 2 nitrogen atoms) and an imidazole (5-member ring with 2 nitrogen atoms) rings which are fused. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Caffeine can be found in 16 different species when the most popular are the coffee species: Coffea arabica and Coffee canephora and the tea plants. It is also found in yerba mate leaves and guarana seeds (both of them produced in South America).
Preparation: Caffeine is mostly obtained from different plants which are cultivated with that purpose. It is calculated that tea or coffee leaves contain a maximum of 5% of caffeine. The caffeine is isolated by extraction using organic solvents and through a high-pressure extraction it is obtained a maximum possible quantity of caffeine. There are a few methods to prepare caffeine in chemical laboratories; these methods include the reaction between dimethylurea and malonic acid.
Physical properties: Caffeine is a white, odorless and hygroscopic crystalline solid. Caffeine tastes bitter and the density is 1.23 g mL-1 and its melting point is 235 ºC and at higher temperatures, it decomposes. It is soluble in water.
Chemical properties: Caffeine is a central nervous system stimulant. It is suspected the mechanism of action involved the reversibly blocks the action of adenosine in some receptors and thus, it is stimulated the nervous system. The caffeine molecule can act in this way because the molecule structure is highly similar to adenosine molecule, particularly on the part corresponding to the nitrogen base adenine.
Uses: Caffeine is highly consumed in the century XXI societies and most of the countries around the world. It is also used largely around the world as drug such as analgesics. The food industry uses the caffeine as additive in many drinks like cola, tea or infusions.
Health effects / safety hazards: Caffeine can be consumed in low doses but it is very toxic at high doses. It is toxic by inhalation or ingestion. It is suspected of cause infertility. It is not flammable. It is probably combustible.
|
Related Links: |
