Calcium carbonate Formula - Calcium carbonate Uses, Properties, Structure and Formula
Calcium carbonate is a widely available natural inorganic compound, also known as limestone, chalk or marble.
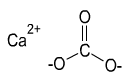
Formula and structure: The chemical formula of calcium carbonate is CaCO3 and its molar mass is 100.1 g/mol. It is a salt made of the bivalent calcium cation (Ca2+) and the bidentate carbonate anion (CO32-), in which the carbon is attached to two oxygen atoms through single bonds and one oxygen atom through a double bond.
Occurrence: Calcium carbonate occurs naturally in several mineral forms including the pure calcite, aragonite and vaterite minerals as well as the impure minerals-limestone, chalk, marble and travertine. It is also the main chemical constituent of eggshells, sea shells, oyster shells, snail shells, corals, etc.
Preparation: Calcium carbonate is mainly obtained from its various natural mineral sources by mining and processing. It is also prepared by chemical synthesis by reacting quicklime (calcium oxide, CaO) with water to give calcium hydroxide (Ca(OH)2), which is then treated with carbon dioxide to precipitate the calcium carbonate salt.
CaO + H2O → Ca(OH)2
Ca(OH)2 + CO2 → CaCO3 + H2O
Physical properties: Pure CaCO3 is an odorless, fine white powder. It has a density of 2.71 g/mL and melting point of 1,339 °C, as its calcite form. The other common mineral form, aragonite, has a density of 2.83 g/mL and melting point of 825 °C.
Chemical properties: Calcium carbonate is insoluble in water and stable at normal temperatures. When heated to high temperatures, it decomposes to form calcium oxide with the release of carbon dioxide.
CaCO3 → CaO + CO2
CaCO3 also releases carbon dioxide when it is reacted with acids. Calcium carbonate reacts with water containing carbon dioxide to form the water soluble calcium bicarbonate salt (Ca(HCO3)2).
Uses: The main applications of calcium carbonate are in building materials, ceramic tiles, blackboard chalk, iron ore purification, oil well drilling fluids, paints, adhesives, and sealants. It also has several medical uses such as antacid, calcium dietary supplement, pharmaceutical filler in tablets, and hemodialysis treatment. It is also used as a food preservative and in toothpastes.
Health effects/safety hazards: Calcium carbonate is not toxic and is safe for consumption. However, excess calcium intake can lead to hypercalcemia or milk-alkali syndrome, which include symptoms like abdominal pain and vomiting, and can be fatal in serious cases.
|
Related Links: |
