Calcium hydroxide Formula - Calcium hydroxide Uses, Properties, Structure and Formula
Calcium hydroxide is an inorganic compound used for many purposes. It is also called slaked lime, and its aqueous solution is called limewater.
Formula and structure: The chemical formula of calcium hydroxide is Ca(OH)2 and its molar mass is 74.09 g/mol.
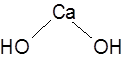
This ionic compound has the calcium metal cation bonded to two hydroxide anions. In the solid form, calcium hydroxide exists in a polymeric structure facilitated by hydrogen bonding between the layers.
Occurrence: Calcium hydroxide occurs naturally, but rarely, in its mineral form as portlandite, which is found in some volcanic and metamorphic rocks.
Preparation: Calcium hydroxide is produced commercially by reacting calcium oxide (lime or quicklime) with water:
CaO + H2O → Ca(OH)2
It is also prepared on smaller scales by the reaction between aqueous calcium chloride and sodium hydroxide.
Physical properties: It is obtained as colorless crystals or a white powder with a density of 2.21 g/cm³ and melting point of 580 °C.
Chemical properties: Calcium hydroxide is relatively soluble in water. It partially dissolves in water to produce a solution called limewater, which is a moderate base. Limewater or aq. Ca(OH)2 reacts with acids to form salts, and it can attack some metals such as aluminum. Limewater readily reacts with carbon dioxide to form calcium carbonate, a useful process called carbonatation:
Ca(OH)2 + CO2 → CaCO3 + H2O
Uses: Calcium hydroxide has many industrial uses, such as in the Kraft paper process, as a flocculent in water and sewage treatment, in the preparation of ammonia, and as a pH modifier. It is also an important ingredient in cement, plaster and mortars. As a fairly non-toxic and mild base, it has many uses in the food industry, including pH adjustment, calcium fortification, digestion aid, and baking soda substitute.
Health effects/safety hazards: Calcium hydroxide is not toxic at low concentrations. However, at higher concentrations, eye/skin contact, inhalation or swallowing of the aqueous calcium hydroxide solution can cause blindness, severe skin irritation, chemical burns, or lung damage.
|
Related Links: |
