Carbon dioxide Formula
Carbon dioxide, also known as carbonic acid gas or carbon anhydride, is an inorganic gas formed as a product of the respiration of plants and animals.
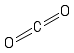
Formula and structure: The carbon dioxide chemical formula is CO2. The molar mass is 44.01 g/mol. The molecule is formed by one carbon cation C4+ and two oxygen anions O2-. The two anions are bound to the carbon cation trough double bonds (pi bonds) making the carbon dioxide as a linear and rigid molecular. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Carbon dioxide is largely found in nature. It is the product of the respiration process. All forms of live need respiration to survive, in this process the organisms inhale or capture oxygen from the environment and through different biochemical reactions; it is produced CO2 and water. Carbon dioxide is found in the atmosphere and is also emitted by volcanoes, natural gas, crude oil and hot springs.
Preparation: Carbon dioxide is produced by the natural and unnatural ways. In the natural way, the carbon dioxide is produced during the respiration of organisms (plants, animals, fungi, bacteria):
C6H12O6 (s) + 6 O2 (g) → 6 CO2 (g) + 6 H2O (l) + heat
The carbon dioxide produced is used for the plants during the photosynthesis in a reverse process that produces oxygen.
In a unnatural way, carbon dioxide can be produced from the combustion of fuels as methane, gasoline, kerosene, propane, minerals, rocks and coal.
CH4+ 2 O2→ CO2+ 2 H2O
Physical properties: Carbon dioxide is a colourless and odourless gas. When concentrate, it can have a acidic odour. The density 1.56 g/mL. Carbon dioxide melting point is -56.6 °C and above this temperature, it sublimates. Carbon dioxide solubility in water is 1.45 g/L, and it is insoluble in ethanol and organic solvents.
Chemical properties: One of the most important reactions for the environment is the solubility of carbon dioxide in water. Even when the solubility is low, this factor is very important for the water bodies in the planet. When the carbon dioxide is in contact with water, it forms carbonic acid, which could decrease the pH of water, causing a problem for aquatic life.
CO2 + H2O → H2CO3
Uses: Carbon dioxide is used in the productions of other most valuable products in the chemical industry. Some of these products are the urea, used as fertilizer and the solvent and chemical precursor methanol. Other products are the carbonate salts as sodium carbonate and bicarbonate (baking soda). It is used as a food additive and also as a propellant. It is present in sodas and soda water and dry ice.
Health effects / safety hazards: Carbon dioxide inhalation can cause headache and increase of the respiration rate. It can cause unconsciousness at high concentration. The dry ice can cause cold burns. It is not flammable.
|
Related Links: |
