Cellulose Formula
Cellulose is an organic compound found mostly in the cell walls of plants. It is extensively used in to produce paper and paperboard.
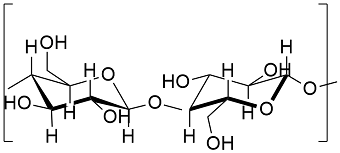
Formula and structure: The cellulose chemical formula is C6H10O5 and its molar mass is 162.14 g mol-1. The molecule is a polysaccharide, which is formed by many D-glucose units bounded by glycosidic bonds (a kind of covalent bond that is mostly in sugars). Thus, cellulose is one of the most extended natural polymers. The final structure of cellulose depends on the micro-organism which produces it. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Cellulose is highly abundant in nature. It is present on the cell wall of green plants, many algae and other micro-organisms such as oomycetes or bacteria. Cellulose is found in cotton, wood and paper.
Preparation: Cellulose is a complex structure, which has not been reproduced through synthetic methods. It is only produced by plants and other organism, thus is is indispensable for the paper industry to use trees and plants to produce the paper pulp.
Physical properties: After the treatment and the extraction of cellulose form the tree and plants, it is a white powder. Its density is 1.5 g mL1- and its melting point is 260 ºC and at higher temperatures, it decomposes. Cellulose is insoluble in water and most organic solvents.
Chemical properties: Cellulose is an organic polymer, which has thousand D-glucose units bounded through hydrogen bonds (the hydrogen bonds are on the most common and essential bonds in nature) that are formed between the oxygen atom of the one hydroxyl group of the glucose and the hydroxilic hydrogen atom of the next glucose unit. The number of D-glucose units depends on the type of plants or the type of material: wood, cotton, etc.
Uses: Cellulose has a highly demanded by the paper industry, which uses it to produce paper, paperboard and card stock. It is also used in the fibers and textile productions, especially of cotton and rayon. Cellulose is the material which is used to fill many chromatographic columns to do chemical assay by HPLC. In the last years it has also been used to produce biocombustibles.
Health effects / safety hazards: Cellulose can irritate mucous and eyes but it is not toxic. It is combustible and should be maintained isolated from flammable materials or heat source.
|
Related Links: |
