Chloric Acid Formula
Chloric acid or chloric (V) acid is a inorganic acid used as reagent and reactive to prepare some chlorate salts and other chemical products.
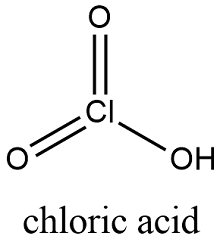
Formula and structure: The chloric acid chemical formula is HClO3 and the molar mass is 84.46 g mol-1. This salt is formed by one proton (H+) and one chloric anion (ClO3-). The chloric anion has a pyramidal-like structure, with a chlorine center atom to which two oxygen atoms are bound through double bonds and 1 oxygen atom is bound in a simple bond. The chlorine atom also has a electron pair that repealed the oxygen atoms and form the pyramidal structure.Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Chloric acid is not found in nature. It is prepared following the methods described below.
Preparation: Chloric acid can be prepared through two different methods:
- By the reaction of barium chlorate and sulfuric acid, following by the filtration of the barium sulfate formed:
Ba(ClO3)2 + H2SO4 → 2 HClO3 + BaSO4
- By heating the hypochlorous acid to additional produces hydrogen chloride:
3 HClO → HClO3 + 2 HCl
Physical properties: Chloric acid is a colorless liquid. Its density is 1 g mL-1. It is soluble in water. It is an oxidant and corrosive.
Chemical properties: Chloric acid is very reactive, it can easily reacts oxidizing many compounds. It is a strong acid, which means in water solution it is found in its deprotonated form:
HClO3 + H2O → H3O+ + ClO 3-
In cold water, it can form other chlorine acids and chlorine species:
8 HClO3 → 4 HClO4 + 2 H2O + 2 Cl2 + 3 O2
3 HClO3 → HClO4 + H2O + 2 ClO2
Uses: Chloric acid is used to prepare chloride salts and other chlorine species. It is also an oxidizer agent.
Health effects / safety hazards: Due to its oxidizer characteristic, it should be used very careful and must avoid the contact with other chemical compound that can react easily with. Chloric acid is a strong acid, thus it can cause severe eyes and skin damage.
|
Related Links: |
