Copper (I) Sulfate Formula
Copper (I) sulfate, also known as cuprous sulfate or diccoper sulfate, is an inorganic salt not largely used in the chemistry industry but still found some application in chemical laboratories.
Formula and structure: The copper (I) sulfate chemical formula is Cu2SO4. The molar mass is 223.15 g/mol. The molecule is formed by two copper (I) cation Cu+ and one sulfate anion SO42-. The molecule is formed by a central sulfate anion to which the two copper cations are joined through a bond Cu-O. The molecule is orthorhombic. Its chemical structure can be written as below, in the common representations used for organic molecules.
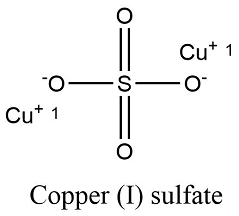
Occurrence: Due to its instability, it is not found freely in nature.
Preparation: Copper (I) sulfate can be prepared from the reaction between the copper (I) oxide and the DMSO (dimethyl sulfate):
Cu2O + (CH3O)2SO2 → Cu 2SO4 + (CH3)2O
Physical properties: Copper (I) sulfate is a white solid with a density 3.6 g/mL. The melting point is 110 °C and above this temperature, it decomposes. Copper (I) sulfate decomposes in water.
Chemical properties: Copper (I) sulfate is not largely used for chemical application due to its large instability. In general, this salt decomposes to form the most stable copper (II) sulfate CuSO4, which has more chemical applications as described below.
Uses: There are not chemical applications described for the copper (I) sulfate, except some chemical reactions in small scale, where it can act as a catalyst. The most common and stable copper (II) sulfate can be used as a catalysts, as an additive in fertilizers and in food and pharmaceutical preservatives.
Health effects / safety hazards: Iron (I) sulfate is very toxic for the aquatic life. It is dangerous if ingested and can cause skin and eyes irritation. It is not flammable. It does not react with other chemical compounds.
|
Related Links: |
