Copper (II) Sulfate Formula
Copper sulfate, also known as bluestone or blue vitriol, is an inorganic salt used as dyeing agent, as catalyst in some organic reaction and mainly as a fungicide to treat fruits and vegetable diseases.
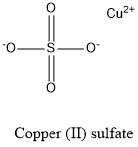
Formula and structure: Copper (II) sulfate chemical formula is CuSO4 and the molar mass is 159.60 g mol-1. The compound is commonly found as a hydrated salt with between 1 to 5 molecules of water. The most common hydrated form is the CuSO4.5 H2O. The pentahydrated form has a molecular mass of 249.69 g mol-1. The structure of the anhydrous salt is formed by one cation Cu2+ and one anion SO42-. Regarding to the pentahydrated salt, it is formed by a complex in which the copper cation is in the center and is surrounded by four water molecules. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Copper (II) sulfate is found in nature as a natural pesticide. It is found in plants, soil and water. However, it is not produced in enough amounts to be used commercially.
Preparation: Copper (II) sulfate is produced through the redox reaction of copper metal and concentrated sulfuric acid in high temperatures:
Cu + H2SO4 → CuSO4
In this reaction the copper metal is oxidized to the cation Cu2+. It can also be prepared by the reaction of copper with diluted sulfuric acid and air.
Physical properties: Copper (II) sulfate is a white powder in its anhydrous form and a blue crystalline solid when it is pentahydrated. Copper (II) sulfate anhydrous and pentahydrate density are 3.60 and 2.28 g mL-1. The melting point of the anhydrous salt is 110 °C, while the pentahydrate decomposes. Above 520 ºC the anhydrous salt decomposes. Copper sulfate is soluble in water and methanol and insoluble in ethanol and acetone. The flame test for copper sulfate emits a green flame.
Chemical properties: Cupper (II) sulfate is a toxic compounds and its toxicity allows its use in the control of fungi, parasite and bacteria. Also fishes and algae can die in presence of copper sulfate. Copper sulfate also has the properties of acting as oxidizing agent of sugars and proteins, so that, it has been used in various test as the Fehling's solution, Benedict's solution or Buriet reagent for reducing sugars and proteins.
Uses: Copper (II) sulfate is mainly used as fungicide and herbicide due to its toxicity. It is used for example to treat fruits as melons and berries to control fungus and parasites. In organic synthesis it is using as a dryer agent and also as a catalyst in some reactions. Copper sulfate has been used as a dye in some vitrals and paints in past, and today its use as coloring ingredients in paints still remains.
Health effects / safety hazards: Copper (II) sulfate is toxic to aquatic life in high concentrations. It can produce toxic flames with mixture with chlorates. It is not harmful for humans in low concentrations.
|
Related Links: |
