Dalton's law Formula
Definition: The Dalton's law is also a law for explaining the behavior of gases and more specifically, for mixture of gases. It was published in 1802 by the English chemist John Dalton. The laws states that when there is a mixture of inert gases (they are not reacting between them), the total pressure exerted is equal to the sum of the partial pressure of the individual gases.
Formula: for a mixture of n gases, the total pressure is:
Ptotal =P1 + P2 + P3 + ... + Pn
Where P1, P2, P3, ..., Pn represent the partial pressure of each gas in the mixture. The Dalton's law is related to the ideal gas law through the next equation:
P = nRT/V → 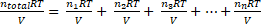 as the term RT/V is common and he same for all the gases, the expression can be reduced to nTotal = n1 + n2 + n3 + ... +nn
as the term RT/V is common and he same for all the gases, the expression can be reduced to nTotal = n1 + n2 + n3 + ... +nn
So, the number of the moles in a mixture of gases is equal to the sum of the moles of each gas.
Use: Calculation of mixtures of gases and the volume and pressure of each gas are very important for the chemical industries. Today the industries use sophisticated software for calculating these parameters, however the Dalton and Avogadro's law are the base of all these technologies.
Example: In a mixture of nitrogen gas, argon gas and helium gas was measure the pressure and it was estimated in 2 atm. The technician also confirmed the pressure of the nitrogen was 0.8 atm and the pressure of helium was 0.5 atm. What is the pressure of argon gas in the mixture?
Ptotal - Pnitrogen - Phelium = Pargon → Pargon = 2 atm - 0.8 atm - 0.5 atm = 0.7 atm
Considerations: At the same that Avogadro's law, currently and for industrial purposes, there are optimizations that should be made in this ideal gas consideration. The expression for the ideal gas does not consider factor that equations as the Van der Waals equation for real gases started to add in order to expain better the real behavior of some gases.
|
Related Links: |
