Dimethylformamide Formula
Dimethylformamide, also known as N,N-Dimethylmethanamide, is an organic solvent used in the chemical industry to manufacture fibers, films and coatings.
Formula and structure: The dimethylformamide chemical formula is C3H7NO and it molar mass is 73.10 g mol-1. The extended formula is CHON(CH3)2 and the molecule is formed by an amide group O=CH-N-R and the nitrogen has two methyl groups bond. The molecular is planar, because even when the nitrogen does not have a double bond (which needs a planar sp2 hybridization); there are some resonance structures that show the nitrogen needs a sp2 conformation (see the structures below). Its chemical structure can be written as below, in the common representations used for organic molecules.
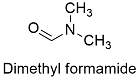
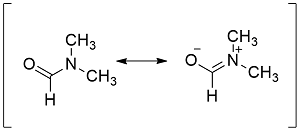
Occurrence: The natural occurrence of dimethylformamide (DMF) has not been reported.
Preparation: The synthesis of dimethylformamide is performed using N,N-methylamine and a solution of sodium methoxide; them a stream of gas carbon monoxide is passed through the solution at 50-150 ºC. Methanol is used as catalyst of the reaction.
Physical properties: Dimethylformamide is a clear to colorless liquid with a fishy ammoniacal. Its density is 0.948 g mL-1. The melting point is -60.5 ºC, and the boiling point is 152-154 ºC. It is miscible in water and most of the organic solvents.
Chemical properties: Dimethylformamide is an excellent solvent. It can be hydrolyzed by strong acids and bases to form a carboxylic acid and an amine. DMF can also react through a decarbonylation to produce dymethylamine. Dimethylformamide reacts with several chemical compounds such as: alkaline metals, azides, hydrides, bromine, chlorine, magnesium nitrate, etc. It also can catalyze thesynthesis of acyl halides.
Uses: Dimethyl formamide is used mostly in the manufacture of fibers, plastics, coatings and acrylic products and some adhesives, rubbers and films. Dimethyl formamide is a solvent used to prepared several medicaments by the pharmaceutical industry. Moreover, some oils processes use the DMF to recover olefins. In laboratories, it is used as solvent to analysis of NMR spectroscopy.
Health effects / safety hazards: Dimtehylformamide is extremely dangerous and toxic by ingestion. It can also irritate skin, eyes and respiratory tract by inhalation. It is suspected to cause cancer by long-term exposition. DMF is flammable and it produces toxic fumes in a fire.
|
Related Links: |
