Dimethylsulfoxide Formula
Dimethylsulfoxide (DMSO) is an organic compound, also known methylsulfinylmethane, is an organic compound largely used in chemical, pharmaceutical industries and in lab-scale as solvent.
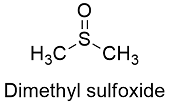
Formula and structure: The dimethylsulphoxide chemical formula is C2H6OS and its extended formula is (CH3)2SO. Its molar mass is 78.13 g mol-1. The molecule is formed by a central sulfur atom that is bond to two methyl groups through single bonds and a oxygen atom, which is joined through a double bond to the sulfur atom. The molecule is planar has a trigonal pyramidal molecular geometry, with a base formed by the 4 atoms and axes constituted by the electron pairs of the sulfur. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Dimethylsulfoxide is found in some plants and fruits in small quantities.
Preparation: The dimethylsulphoxide is found in nature in low levels, thus, it should be prepared through several methodologies to be used in industries. The first strategy is the preparation through the catalytic oxidation of dimethyl sulfide with oxygen or nitrogen dioxide at 105 ºC. It is also obtained as a by-product of the paper manufacture and with the oxidation of dimethyl sulfide with nitrogen tetroxide,
Physical properties: Dimethyl sulfoxide is a colourless liquid with a pungent odor. Its density is 1.1004 g mL-1 and the melting and boiling points are 19 ºC and 189 ºC. It is completely miscible in water and other polar solvents and interestedly it is also soluble/miscible in non-polar solvents such as diethyl ether.
Chemical properties: Dimethyl sulfoxide is a polar aprotic solvent, which means the molecule has a polarity due to the difference of the electronegativity between the atoms which form the molecule; but it does not have any hydrogen in the molecule, thus it cannot form hydrogen bonds with other compounds. There a range of reactions which can be performed using DMSO, attack to electrophiles by the nucleophiles (sulfur or oxygen): (CH3)2SO + CH3I → [(CH3)3SO]I. The DMSO can also be used as oxidant in reactions such as the Swern oxidation.
Uses: Dimethyl sulfoxide is used as solvent and reactant of many solutions. In the pharmaceutical industry it is used as vehicle for topical application and also as analgesia and anti-inflammation. It is particularly used due to the capacity of penetration cell membranes of the DMSO. Nowadays, it has been used used in the manufacturing of microelectronic devides and in some biochemical reactions such as polymerase chain reaction (PCR).
Health effects / safety hazards: Dimethyl sulfoxide can cause skin and eyes irritation and also respiratory tract irritation. It is not flammable.
|
Related Links: |
