Equilibrium constant Formula
Definition: The Equilibrium constant, called K or Keq, defines the state in which reactants and products concentration of a reaction have no change over the time.
A reversible chemical reaction can follow in the forward or reverse directions. The formation of the products is favorable when the reaction follows the forward direction and is unfavorable when the reaction goes on the reverse direction to the formation of the reactants. During the equilibrium, the rate of the forward reaction equals to the rate of the reverse reaction and reactant and product concentration are constant. It is important to notice, to keep the concentrations at equilibrium no means the concentration are equal, instead they value at different each other but not vary.
Formula: For a reaction as the next one:
aA + bB ⇌ cC + dD
where, a, b, c and d are the stoichiometric coefficients and A and B are the reactants and C and D are the products of the reaction. The double arrow means the reaction is reversible. The equilibrium constant is written as:
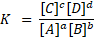
Where [C], [D], [A] and [B] are the concentration of each chemical compound in the reaction. If the reactants or products are gases, the concentration can be substituted by the term partial pressure of the gases (P), so that:
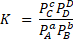
Use: The value of the equilibrium constant is useful for determining the concentration or pressure of all the reactants and products when the equilibrium is reached.
Example: An example of calculation of the equilibrium constant is:
2SO2(g) + O2(g) ⇌ 2SO3(g)
[SO2] = 0.62 M; [O2] = 0.45 M; [SO3] = 0.92 M
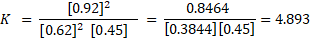
Considerations: The equilibrium constant value gives an idea about which components are mostly being formed during the equilibrium. A K number ~ 1000 or more is very large and it means there are mostly products during the equilibrium. A K number ~ 0.001 or less means there are mostly reactants during the equilibrium. If K is > 0 < 1000 means there are both, reactants and products, at the equilibriums.
|
Related Links: |
