Ethane Formula
Ethane is a gas, largely found in natural gas, and used in the mixtures with other gases to produce combustibles.
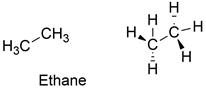
Formula and structure: The ethane chemical formula is C2H6 and is also written as Et. Its molar mass is 30.07 g mol-1. The molecule is the second simplest organic compound after the methane and it is formed by 2 carbon atoms bound to 3 hydrogen atoms. The carbon atoms have a sp3 hybridization (tetrahedral geometry), so that the molecule bonds are free rotation. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Ethane is the second larger compound present in natural gas. Similar to methane, it can be extracted from natural gas reserves or oil wells. Ethane is present in the atmosphere and seas.
Preparation: The commercial available ethane is not produce by synthesis, instead it is recovered from distillation of crude petroleum or other natural gas sources.
Physical properties: Ethane is a colorless and odorless gas. The ethane melting and boiling point are -182.79 ºC and -88.6 ºC, respectively. Its density varies with the temperature, thus it is 0.545 g mL-1 at -89 ºC and 1.36 g mL-1 at 0 ºC. Ethane, as methane and propane, can easily ignite forming vapors lighter than air. It is not soluble in water, but is soluble in ether, ethanol, ether and benzene.
Chemical properties: Similar to methane, ethane molecule is formed by a simple chain of two carbons atoms. The dipole moment in the molecule is 0 and it is not reactive when compared with molecules with functional groups such as hydroxyl or alkenes and alkynes. When compared to the homologous alkene, ethene, it can be observed while ethene participates in a wide quantity of reactions due its pi electrons, the ethane promote a few reactions by itself.
Uses: Ethane- propane mixtures are very used as combustible. This mixture is shipped as a liquid compressed gas. The chemical properties of ethane results in a few reactive specie to be used by the chemical industry as precursor in organic synthesis.
Health effects / safety hazards: Ethane may cause asphyxia by the displacement of air because it is heavier than air. It is a very flammable gas and can easily ignite. Methane can also cause explosion when is expose to high temperatures or fire. It is not compatible with oxidizing agents.
|
Related Links: |
