Ethanolamine Formula
Ethanolamine or 2-aminoethanol (abbreviated as ETA) is a viscous and hygroscopic organic base. Ethanolamine is part of amino alcohols group.
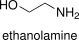
Formula and structure: The chemical formula of ethanolamine is H2NCH2CH2OH or NH2CH2CH2OH. Its molecular formula is C2H7NO and its molar mass is 61.08308 g mol-1. Ethanolamine is a base formed by an amine and a hydroxyl group. The ethanolamine structure is formed by a primary amine (-NH2) and a primary alcohol (-OH). Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Ethanolamine is present in biological membranes, especially in the phospholipids, where us the second-most-abundat head group. ETA is biosynthesized from L-serine through serine decarboxylases enzymes (SDC) in a reaction that also produces CO2:
phosphatidyl-L-serine → phosphatidylethanolamine + CO2
HOCH2CH(CO2H)NH2 → HOCH2CH2NH2 + CO2
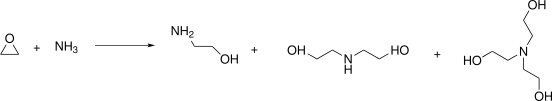
Preparation: Ethanolamine is produced through the reaction of ethylene oxide with aqueous ammonia. The secondary products are diethanolamine and triethanolamine.
Physical properties: Ethanolamine is a viscous, hygroscopic, colorless to yellow pale liquid with ammoniacal odor. Its melting and boiling points are 10 ºC and 170 ºC respectively. Its density os 1.012 g mL-1 and it is miscible with water.
Chemical properties: Ethanolamine reacts with organic and inorganic acids and other chemical compounds as acetic anhydride, acrolein, acrylonitrile, epichlorohydrin, vinyl acetate and others. Similarly to other amines, ethanolamine combustion produces toxic fumes of nitrogen are emited.
Uses: Ethanolamine is used as surfactant in the production of detergents and emulsifiers, chemical intermediaries and pharmaceutical products. ETA is used for buffering emulsions in order to regulate the pH in chemical fine compounds. Other important ethanolamine use is as Sclerosing agent in the scleritherapy of varicose veins, where a solution of Ethamolin (ethanolamine oleate) is injected into the blood vessels. Moreover, ethamolin may be used as a treatment of hemorrhoids.
Health effects/safety hazards: Ethanolamine is moderately toxic but the fumes that are emitted where ETA is heated to decomposition are highly toxic. TEA is corrosive to skin and it may attack copper and rubber. Ethanolamine is flammable, hygroscopic and incompatible with strong oxidizing agents.
|
Related Links: |
Related Topics
Normality Formula
