Ferrous sulfate Formula
Ferrous sulfate, also known as iron (II) sulfate, is an inorganic salt that is used as precursor to obtain iron compounds. This salt was used in the Middle Age as copperas and green vitriol.
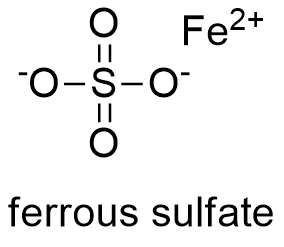
Formula and structure: The ferrous sulfate chemical formula is FeSO4. Generally, the ferrous sulfate is mostly present in nature as hydrated salts; the table 1 shows the main ferrous sulfate salts. All the ferrous sulfate salts have octahedral crystalline structure.
|
Number of water molecules |
Chemical formula |
Molar mass (g mol-1) |
|
0 → anhydrous salt |
FeSO4 |
151.908 |
|
1 → monohydrated salt |
FeSO4.H2O |
169.923 |
|
4 → tetrahydrated salt |
FeSO4.4H2O |
224.120 |
|
5 → pentahydrated salt |
FeSO4.5H2O |
242.135 |
|
7 → heptahydrated salt |
FeSO4.7H2O |
278.075 |
The chemical structure for the anhydrous ferrous sulfate can be written as below, in the common representations used for organic molecules.
Occurrence: Ferrous sulfate is mainly present in nature as heptahydrated salt. However, the other hydrated salts are also found in nature constituting some minerals.
Preparation: Anhydrous ferrous sulfate is obtained from the reaction between elemental iron and sulfuric acid, to yield ferrous sulfate and hydrogen gas (reaction I) or by oxidation of pyrite (reaction II).
Fe + H2SO4 → FeSO4 + H2 (reaction I)
2FeS2 + 7O2 + 2H2O → 2FeSO4 + 2H2SO4 (reaction II)
Physical properties: Ferrous sulfate in their different hydrated states are turquoise or blue-green, odorless and crystal solid. Anhydrous ferrous sulfate has a melting point of 680 ºC, however it decomposes over 300 ºC. Its density is 1.898 g mL-1. All ferrous sulfate salts are soluble in water.
Chemical properties: Ferrous sulfate salts tend to lose or gain water molecules depending of the medium. In water, ferrous sulfate salts are hydrolyzed, forming the aquo complex [Fe(H2O)6]+2. By the other side, these salts lose water molecules when are in contact with the air. In humidity ambient, ferrous sulfate salts oxide to ferric sulfate.
Uses: in medicine, ferrous sulfate is a supplement of iron in human body. Thus, it is used to treat anemia and it is suministrated to pregnant women as iron source. Ferrous sulfate was used for many centuries to manufacture inks and wool/fabric dyeing; particularly it was used to obtain indigo dye. Similar to ferric sulfate, ferrous sulfate is used to purify wastes by flocculation.
Health effects/safety hazards: Ferrous sulfate causes irritation of respiratory tract. It has a low toxicity; however, the ferrous sulfate pure or in concentrated solution, is very dangerous and can causes diarrhea or even death by damaging blood vessels.
|
Related Links: |
Related Topics
Free Math worksheets, Free phonics worksheets, Math Games and ...
