Hydrogen Phosphate Formula
Hydrogen phosphate, also known as monohydrogen phosphate, is an inorganic ion found in nature with biological function. In industry, it is used as a food additive.
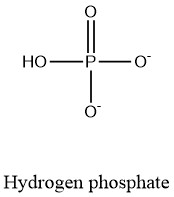
Formula and structure: The hydrogen phosphate chemical formula is [HPO4]2-. The molar mass is 95.97 g/mol. The ion is formed from the loss of two protons H+ of the phosphoric acid H3PO4, which explains the charge of 2 exhibits by this ion. After the deprotonation, the ion hydrogen phosphate remains as one proton H+ bound to one phosphate anion PO43-. Its chemical structure can be written as below, in the common representations used for molecules.
Occurrence: Hydrogen phosphate ion is found in biological system, forming part of buffers and essential mechanism of living organisms.
Preparation: Hydrogen phosphate is formed through the dissolution in water of the phosphoric acid, which establishes chemical equilibrium (see on chemical properties below).
Physical properties: The hydrogen phosphate ions physical properties are directly defined by the cation to which are bound to form the different phosphate salts. Thus, it is not possible to define the physical properties of the ion isolated. Many of the phosphates salts that can be formed with different cations are white solid highly soluble in water.
Chemical properties: Hydrogen phosphate ion shows an structure formed by a centered phosphorous atom to which three oxygen atoms are bounded, one of this oxygen has an hydrogen attached, forming an hydroxyl group. This group is well known to be able to form hydrogen bond, explaining the high solubility in a polar protic solvent as water. As second characteristic of this ion, it is necessary to comment that a group of reactions can be formed when the phosphoric acid is dissolved in water, so not only the hydrogen phosphate is formed and instead there are found different acid-base equilibria:
H3PO4 ⇌ H2PO4- + H+
H2PO4- ⇌ HPO42- + H+
HPO42- ⇌ PO43- + H+
Uses: Hydrogen phosphate has important roles in micro-organisms; an example is through the formation of important metabolites in Saccharomyces cerevisiae. In the form of salts as the sodium hydrogen phosphate is used as a food additive and a acid regulator in food industry.
Health effects / safety hazards: Hydrogen phosphate is not toxic and not flammable. It does not react with other chemical compounds.
|
Related Links: |
