Hydroiodic Acid Formula - Hydroiodic Acid Uses, Properties, Structure and Formula
The aqueous solution of hydrogen iodide is called hydroiodic acid, and it is the most acidic among the hydrohalides.
Formula and structure: The chemical formula of hydroiodic acid (aq. hydrogen iodide) is HI. Its molar mass is 127.91 g/mol. Hydrogen iodide is the gaseous form, while hydroiodic acid is the aqueous solution of HI. They are both interchangeable. HI is a simple diatomic molecule with the below structure:

Hydrogen iodide is polarized because of the electronegativity of the iodide. Due to the large size of the iodide ion, the negative charge is dispersed resulting in a weaker H-I bond. This allows the H+ to be easily dissociated and hence, HI is a stronger acid than HCl, HBr and HF.
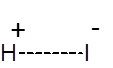
Preparation: Hydroiodic acid is prepared commercially by the reaction of iodine (I2) with hydrazine, giving hydrogen iodide and nitrogen gas.
Hydroiodic acid is also prepared by bubbling hydrogen sulfide gas through an aqueous solution of iodine.
At the end of the reaction, HI is distilled to give hydroiodic acid in the desired concentrations.
Physical properties: Hydrogen iodide is a colorless gas with an acrid odor that is readily soluble in water to give hydroiodic acid. "Concentrated" hydroiodic acid is usually 48-57% HI in water. Its exact physical properties (boiling point, melting point and density) depend on the concentration of HI in the aqueous solution.
Chemical properties: Hydroiodic acid is a strong and reactive acid. It should be used carefully due to its powerful reactivity. It can react violently with metal powders, ammonia, etc., to generate fire and explosions. It is highly corrosive, and reacts strongly with bases. HI also decomposes on heating to generate toxic fumes, and gets oxidized rapidly in air.
Uses: One of the most common uses of HI is to form alkyl iodides, an important class of organic compounds, by reacting HI with alkenes or primary alcohols. Hydroiodic acid is also a common reducing agent for various industrial purposes.
Health hazards/ health effects: The HI gas is toxic by inhalation and highly irritating to skin, eyes and mucous membranes. Hydroiodic acid can cause severe skin burns and eye damage, and is highly toxic if inhaled, ingested or absorbed through skin. Long-term exposure in low concentrations can also cause adverse health effects.
|
Related Links: |
