Lactic acid Formula
Lactic acid, also known by the IUPAC name 2/hydroxypropanoic, is an organic acid biosynthesized as intermediated of sugar metabolism in body. It is also used by food industry as preservative.
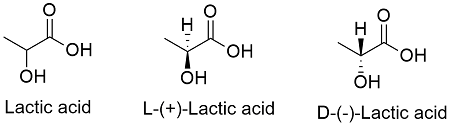
Formula and structure: The lactic acid chemical formula is C3H6O3 and its extended chemical formula is CH3CH(OH)CO2H and its molar mass is 90.080 g mol-1. The molecule is classified as an alpha-hydroxy acid due it has a hydroxyl group (-OH) and a carboxylic group (-COOH) join to the same carbon atom. This central carbon is chiral and the other 2 substituent groups are a hydrogen atom an a methyl group (-CH3), so that there are two possible structures: the L-(+)-Lactic acid and the D-(-)-Lactic acid. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Lactic acid is produced naturally in muscles during exertion, through the conversion of pyruvate catalyzed by the enzyme lactate dehydrogenase. This reaction is used in biotechnology industry on the process known as lactic acid fermentation. It can also be found in sour milk and other milk derivates such as yogurt and cottage cheese.
Preparation: Lactic acid can be prepared from two strategies: the biotechnological and the synthetic methods. In the biotechnological method, the lactic acid is produced in large scale by the fermentation of carbohydrates (such as glucose, corn syrups, etc) and nutrients (such as peptides and amino acids) by microorganism of the genus Lactobacillus. The fermentation also produces formic and acetic acid. The synthetic preparation of lactic acid is made from acetaldehyde and carbon monoxide, in acid solution at 130-200 ºC.
Physical properties: Lactic acid is a colorless to yellow syrupy liquid or a white powder. Its density is 1.029 g mL-1 and the melting and boiling points are 18 ºC and 122 ºC. It is corrosive to metals and tissue. Lactic acid is soluble in water and ethanol.
Chemical properties: Lactic acid is a weak organic acid, however it can also react in the same way to other stronger acids; the carboxylic acid group presents in the molecule donate a hydrogen ion in the presence of bases (organic or inorganic bases). One of the most relevant characteristics of lactic acid is the double physical state that can present: while the racemic mixture of D,L-lactic acid is a liquid, the enantiopures forms are white powder.
Uses: Lactic acid is used in medicine as a intravenous fluid that works as isotonic to resuscitation after blood loss. It can also be used as a raw material to produce some polymers. Lactic acid is largely use in food industry as a food preservative and flavoring and in chemical industry to manufacture detergent and mosquitoes lure.
Health effects / safety hazards: Lactic acid in high concentration can irritate severely eyes and other mucous membrane. If ingested in high concentration, it may be corrosive. When heated in presence of diazo compound can produce flammable or toxic gases. It can react with sulfites, nitrites and thiosulfates also generating toxic gases. It can corrode many metals.
|
Related Links: |
