Lithium Aluminum Hydride Formula - Lithium Aluminum Hydride Uses, Properties, Structure and Formula
Lithium aluminum hydride (or LAH) is an inorganic compound used as an important reducing agent.
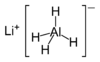
Formula and structure: The chemical formula of lithium aluminum hydride is LiAlH4, and its molar mass is 37.95 g/mol. The chemical structure of LAH is shown below. It exists as a monoclinic crystalline solid, consisting of lithium cation (Li+) and the aluminum hydride anion (AlH4-), in which Al is covalently bonded to the four H atoms.
Preparation: Lithium aluminum hydride is prepared on small scales by reacting lithium hydride with aluminum chloride:
4 LiH + AlCl3 → LiAlH4 + 3 LiCl
The industrial preparation of LAH takes place in two steps. In the first step, sodium aluminum hydride is formed by reacting sodium and aluminum metals with hydrogen under high pressure and temperature. In the second step, this product is reacted with LiCl, to exchange the metal cations (Na for Li) giving the final LAH product:
Na + Al + 2 H2 → NaAlH4
NaAlH4 + LiCl → LiAlH4 + NaCl
Physical properties: LAH is found as an odorless, greyish or white crystalline solid with a density of 0.97 g/mL, and a melting point of 150 °C. It is also available commercially as a dispersion in mineral oil to prevent its violent reaction with atmospheric moisture.
Chemical properties: LAH reacts violently with water to release hydrogen gas:
LiAlH4 + 4 H2O → LiOH + Al(OH)3 + 4 H2
LAH is not very stable and it can decompose over time into Li3AlH6 and LiH, which eventually decompose into LiAl and H2. When heated, LAH decomposes quickly into these same components. LAH is a strong base and a powerful reducing agent. It is soluble in ether and tetrahydrofuran, but sparingly soluble in other organic solvents.
Uses: It is mainly used as a reducing agent in organic synthesis, and a reagent in the preparation of a variety of pharmaceuticals, organic chemicals and metal hydrides (such as NaH). It is also used as a polymerization catalyst and a propellant. It is considered as a potential hydrogen storage agent in fuel cells.
Health effects/safety hazards: LAH is very irritating to the eyes, skin, mucous membranes and respiratory system. The solid can cause severe eye and skin burns. It is very harmful by inhalation. Its violent reaction with water is also a safety hazard.
|
Related Links: |
