Magnesium hydroxide Formula - Magnesium hydroxide Uses, Properties, Structure and Formula
Magnesium hydroxide is a non-toxic inorganic base. Its aqueous suspension is known as milk of magnesia, a common antacid.
Formula and structure: The chemical formula of magnesium hydroxide is Mg(OH)2, and its molar mass is 58.32 g/mol. The chemical structure is shown below, where the central Mg is bonded to the two hydroxyl groups. The compound is ionic, existing mainly as Mg2+ and OH- ions.
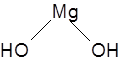
Occurrence: Magnesium hydroxide occurs naturally as the mineral brucite, which is a major source of Mg(OH)2 for many applications.
Preparation: For most industrial applications, Mg(OH)2 is extracted from seawater (Mg salts are always present in seawater). Magnesium chloride in the sea water is reacted with lime (calcium oxide) to form the Mg2+ and OH- ions, which precipitate into magnesium hydroxide salt. Another method is the electrolysis of fused magnesium chloride, which also gives Mg(OH)2 in the same way.
As brucite, it is formed by the alkali-carbonate reaction, in which magnesium carbonate reacts with alkali hydroxides (such as KOH and NaOH).
MgCO3 + 2 NaOH → Mg(OH)2 + Na2CO3
Physical properties: Magnesium hydroxide is obtained as an odorless white solid with a density of 2.34 g/mL, and a melting point of 350 °C. It is also available as an aqueous suspension in water.
Chemical properties: At high temperatures, solid magnesium hydroxide undergoes a very endothermic (absorbs heat from atmosphere) decomposition into magnesium oxide and water, making it a good smoke suppressing and fire-fighting agent.
Mg(OH)2 → MgO + H2O
It is moderately basic and neutralizes mild acids. It readily dissociates in water to give hydroxyl ions and Mg ions. It is soluble in water, but because of this dissociation reaction, it forms a white milky suspension, hence the name (milk of magnesia).
Uses: Due to its mildly basic and non-toxic nature, magnesium hydroxide is widely used as an antacid to neutralize stomach acids and prevent indigestion and heartburn. It is also used as a laxative, antiperspirant, underarm deodorant, to treat sores, in wastewater treatment, and as a fire retardant.
Health effects/safety hazards: Magnesium hydroxide is not considered toxic or hazardous, and it is fit for human consumption in recommended quantities.
|
Related Links: |
