Nitronium perchlorate Formula
Nitronium perchlorate, also known as nitryl perchlorate or nitroxyl perchlorate is an inorganic salt used as oxidizing and nitrating agent in chemical industry.
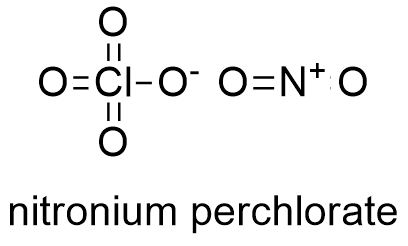
Formula and structure: The nitronium perchlorate chemical formula is NO2ClO4. Its molar mass is 145,4561 g mol-1. Nitronium perchlorate molecule is formed by the anion perchlorate ClO4- and the cation nitronium NO2+. These ions form monoclinic crystals by alternating ClO4- and NO2+ ions in crystal cells. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: similar to other perchlorates, nitronium perchlorate is rarely found in nature and most of them is synthesized by chemical industries. Most of perchlorate in nature is found in Atacama Desert in Chile, where it is in deposits of sodium nitrate.
Preparation: nitronium perchlorate is prepared from dinitrogen pentoxide and perchloric acid anhydrous; the reaction also forms nitric acid, which is immediately dissociated:
N2O5 + HClO4 → NO2ClO4 + H+ + NO3-
Other synthesis route prepares the nitronium perchlorate from nitric acid:
HNO3 + HClO4 → NO2ClO4 + H2O
Physical properties: Nitronium perchlorate is a colorless, odorless and hygroscopic crystal solid. Its melting point is 135 ºC and in temperatures >135 ºC it decomposes. Its density is 2.2 g mL-1.It is slightly soluble in water and other polar solvents.
Chemical properties: nitronium perchlorate is a molecule with a high number of oxygen atoms. In consequence, nitronium perchlorate is a very strong oxidant agent. However, the use of NO2ClO4 is limited because this salt is hygroscopic and its hydrolysis from nitric acid and chloric acid:
NO2ClO4 + H2O → H+ + NO3+ + ClO4-
Uses: Nitronium perchlorate is used in chemical industry to promote oxidation and nitration in organic synthesis. Moreover, it is used as a potent oxidizer for high-energy rocket propellant preparations.
Health effects/safety hazards: Nitronium perchlorate is an irritating agent for eyes and skin. It can easily explode in presence of strong acids, reducing agents or flammable compounds.
|
Related Links: |
