Nitrous oxide Formula
Nitrous oxide, also known as sweet air, hypo nitrous oxide or laughing gas, is a inorganic gas used as anaesthesia and propellant. It is part of the list of the Essential Medicines of the World Health Organization.
Formula and structure: The nitrous oxide chemical formula is N2O. The molar mass is 44.01 g/mol. The molecule is formed by two nitrogen atoms N and one oxygen atom O. The nitrogen anions are bound through a triple bond, and then one of these atoms is bound to the oxygen through a single bond. As second structure, coming from the resonance of electrons, is formed by a centred nitrogen atom, to which oxygen and a second atom of nitrogen are bound through double bonds. The geometry of the molecule is linear. Its chemical structure can be written as below, in the common representations used for organic molecules.
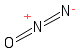
Occurrence: Nitrous oxide is found in nature, it is a component of the atmosphere of our planet. It is also produced by microorganisms during their biochemical processes.
Preparation: Nitrous oxide is produced as emission of gas and side product during the industrial production of nitric acid. It is specifically produced through a reaction of decomposition of ammonium nitrate at high temperatures:
NH4NO3 → 2 H2O + N2O
It can also be prepared from the decomposition of the sodium nitrate and ammonium sulfate at high temperatures. A less used method is the decomposition of the hyponitrous acid at room temperature but in an interval of 2 weeks.
Physical properties: Nitrous oxide is a colourless, with a sweet oudor gas. The density of this gas is 1.977 g/mL. Its melting point is -96.86 °C and its boiling point is -88.48 °C. It is slightly soluble in water but it is soluble in ethanol, ether and sulfuric acid.
Chemical properties: Nitrous oxide shows a low reactivity, it is one of the reasons that allows its use as a medicine. At room temperature, few reaction of nitrous oxide are known. Most of the characterized reactions take place at high temperatures, due to the increment of the kinetic energy of the molecules. One of this reactions is responsible for the use of nitrous oxide as a propellant. Nitrous oxide can react with sodium amide to produce sodium azide and ammonia in exothermic reaction:
2 NaNH2 + N2O → NaN3 + NaOH + NH3
Uses: Nitrous oxide can be used as a propellant in rocket motors, according to the above reaction. It can also be used as a unique component along with some catalysts due to its decomposition at high temperatures is extremely exothermic. Nitrous oxide is also used as an internal combustions in engines. In food products, it is used as a aerosol spray propellant. However, the most popular use of nitrous oxide is as a anaesthetic and analgesic in surgeries, particularly is used by dentists.
Health effects / safety hazards: By inhalation, nitrous oxide can cause faint, euphoria and vomits. However, it is not toxic. It should be used carefully by medical services. It has been found as a greenhouse gas, with contribution to the global warming. It is not flammable and not reactive at room temperature, but its hazards increments with the increment of the temperature.
|
Related Links: |
