Oxygen Formula
Oxygen is both, a compound and an element of the periodic table. As compound, it is a gas and the main component of the air being indispensable to keeping the organisms alive.
Formula and structure: The oxygen chemical formula is O2. The molar mass is 32.00 g/mol. The molecule is diatomic, meaning that is formed by two oxygen atoms that are bound through a double bond. This bond is covalent. Its chemical structure can be written as below, in the common representations used for organic molecules.
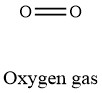
Occurrence: Oxygen is found in the Earth. It is the one of the most abundant element of the Earth. It represents 1/10 part of the atmosphere. It is also present in all the living organisms: bacteria, fungi, plants and animals that need the oxygen for the essential biochemical reactions.
Preparation: Oxygen can be prepared by some chemical reactions in laboratory and in nature. In nature, it is synthesized from CO2, water and sunlight by the plants through the photosynthesis. In laboratories, it is prepared from air, which is passed through a different membrane to separate the oxygen from nitrogen, helium and other gases present in air. Oxygen can also be prepared from liquefaction of air.
Physical properties: Oxygen is a colorless, tasteless and odorless gas. The density is 1.43 g/mL and the melting point is -218.9 and the boiling point is -182.92 °C. It is not combustible but in fire, the oxygen will aggravate it.
Chemical properties: Oxygen is an atomic element in periodic table, but it is impossible to find it in this elemental form. The oxygen has an electronic configuration1s22s22p4, thus, it would need two more electrons to reach the configuration of the noble gas Ne. The reaction to reach the configuration of any noble gas is so favourable that the best configuration of the oxygen is through bounding to a second atom by a double bond reaching both an octet of noble gas.
Uses: Oxygen is used for all the living organisms to accomplish their vital functions. It is also used in the manufacturing of gases as ammonia, methanol and acetylene. It is also used to oxidize products in diverse chemical reactions. Gas cylinders of oxygen are used in medicine to treat patients with breathing syndromes.
Health effects / safety hazards: Oxygen is not toxic at the normal concnetrations find in air, however at high pressures, it can be nocive. It is not flammable but in presence of air, oxygen will contribute to keep the flames.
|
Related Links: |
