Ozone Formula
Ozone, known by the IUPAC name of trioxygen, is an inorganic gas compound of atmosphere. Its lack has been associated to environmental problems due it is the main constituent of ozone shield.
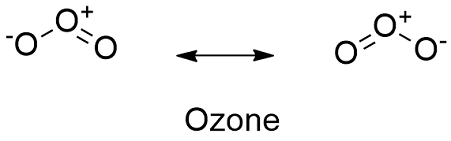
Formula and structure: The ozone chemical formula is O3 and its molar mass is 48.00 g mol-1. The molecule is formed by three oxygen atoms in a structure highly resonant with 1 double bond and 1 single bond and additional 2 partial charges: a negative and a positive. The usual representation of ozone molecule shows it as a "V" structure with an angle of 116.78º between the central carbon atoms and the other two carbons. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Ozone is large found in atmosphere where is formed from molecular oxygen O2 and UV light or electrical discharges. The ozone forms the ozone layer, a gas layer that is 10-50 km above the Earth's surface.
Preparation: Ozone is mostly found in nature, where is produced through a reaction known as Chapman cycle and it consists in the formation of the ozone from molecular oxygen and UV light. In chemical laboratories it is produced by passing air between two electrodes (that have the same role which UV light).
Physical properties: Ozone is a pale blue to colorless gas with a pungent or sharp odor. Its density is 2.144 g mL-1. Its melting point is -192.2 ºC and its boiling point is -112 ºC. Ozone is not soluble in water but it is soluble in carbon tetrachloride and sulfuric acid.
Chemical properties: Ozone is known by its oxidizing power, which remains like one of the most powerful. It can oxidize all metals except gold and platinum (reaction I); nitric oxide (reaction II) and also nitric oxide (III); sulfides compounds (IV):
Cu + O3 → CuO + O2 (I)
NO + O3 → NO2 + O2 (II)
NO2 + O3 → NO3 + O2 (III)
PbS + 4 O3 → PbSO4 + 4 O2 (IV)
Ozone is diamagnetic and also unstable, thus it decomposes easily to oxygen:
2 O3 → 3O2
Use: Ozone is mainly used in industry to promote many oxidations such as described above. It is also used for combustion reactions and in many combustible gases. Ozone is many useful in the water treatment. This is an important component of our atmosphere due the ozone layer protects against the UV light.
Health effects / safety hazards: Ozone can cause skin and eyes irritation. It is fatal by inhalation and ingestion and can also cause genetic defects. Ozone is very toxic to aquatic life. It is a strong oxidant, so that it can or intensifies fire and can react with many chemical substances.
|
Related Links: |
