Percent by volume Formula
Definition: Percent by volume or volume percent is a common expression used for expressing concentration. It is related to the molar concentration but the difference is that the volume percent is expressed with a denominator of 100. It is used for reporting concentration of liquids solutes in solution.
General formula: the general formula for calculating the percent by formula is:
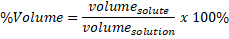
It is also called %V/V and it is always expressed as percentage (%) and the units of the volume should be in mL. Another way to express this % is as the volume of solute in mL that are in 100 mL of solution.
Use: It is a common formula in chemistry. As the same as molar concentration it is used for expressing the concentration of solutes in solution. Percent by volume is also widely use in pharmaceutical field for expressing the concentration of different components in solution.
Example: A solution of propanol (CH3CH2CH2OH) is prepared by dissolving 67 mL in enough water to have a final volume of 250 mL. What is the volume percent of the ethanol?
First, it should be identified
Volume of solute = 67 mL
Volume of solution = 250 mL
% V/V = (67 mL/ 250 mL) x 100% = 26.8%
How many mL of HNO3 concentrate are needed to prepare 250 mL of solution 4%?
First, identify the data available:
Volume of solute =?
Volume of solution = 250 mL
% V/V = 4%
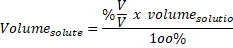
Volume solute = 4% x 250 mL / 100% = 10 mL of HNO3 are needed for preparing
Considerations: The percent by volume is very related to the percent by mass, which is the mass of solute in 100 g of solution or the percent mass/volume, which is the mass of solute dissolved in 100 mL of solution.
|
Related Links: |
