Percent Composition Formula
Percent composition is the term used to describe the percent by mass of each element in a compound. It is typically found using the molar mass values for both the elements in the compound and the compound itself.
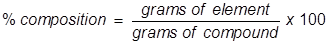
Percent Composition Formula Questions:
1.Find the percent composition of water.
Answer:
First, find the molar mass of water (H2O).
H = 1.01 x 2 = 2.02 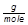 H2O = 2.02 + 16.00 = 18.02
H2O = 2.02 + 16.00 = 18.02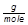
O = 16.00 x 1 = 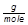
Now find the percent composition of each element.
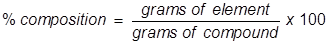
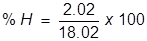
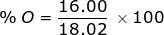
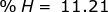
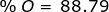
2. Find the percent composition of calcium nitrate, Ca(NO3)2.
Ca = 40.08 x 1 = 40.08 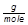 Ca(NO3)2 = 40.08 + 28.02 + 96.00 = 164.10
Ca(NO3)2 = 40.08 + 28.02 + 96.00 = 164.10 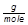
N = 14.01 x 2 = 28.02 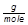
O = 16.00 x 6 = 96.00 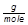
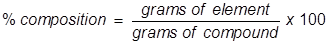
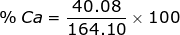
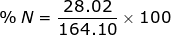
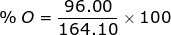
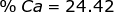
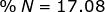
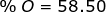
|
Related Links: Percent Composition Quiz |
