Phosphate Ion Formula
Phosphate is an inorganic ion that is present in a large number of salts such as sodium phosphate, cobalt phosphate and others that are used in many fields as agriculture, chemicals and medicine.
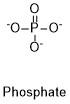
Formula and structure: The chemical structure of phosphate is PO43-. The molar mass is 94.97 g/mol. It is formed by a centered phosphorous atom that is bond to four oxygen atoms, one of the bonds is a double bond and the other three oxygen atoms have simple bonds. The oxygens attached by simple bonds have a negative charge, thus the ion is trivalent and with a tetrahedral geometry. and its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: In nature there are many compounds formed by phosphate joins to other atoms. These compounds are widely extended in the nature, for example in minerals and rocks (iron phosphate, aluminum phosphate, cobalt phosphate), in organisms (sodium and potassium phosphates), where is part of cells and complex molecules and in seawater.
Preparation: Most of the phosphate used in the world is extracted from mineral sources. There are some rocks called phosphate rocks, where it is particularly extracted. There are a few methods for producing phosphates in small scale, but any of them are commercially due to the high cost compared to the mines.
Physical properties: Phosphates are solids and have different colors and physical appearance. Some of them are soluble in water, for example, the phosphates forming with elements of the group I of the periodic table, then potassium, sodium, cesium and rubidium form phosphates soluble in water. Ammonium phosphate is also soluble in water but other phosphates are partially or insoluble in water.
Chemical properties: phosphate comes from the dissociation of the phosphoric acid, and since his acid is trivalent, there are three different species that are forming during the aqueous equilibrium.
H3PO4 ⇌ H+ + H2PO4-
H2PO4- ⇌ H+ + HPO42-
HPO42- ⇌ H+ + PO43-
According to pH of the solution, some of these species can be present in less or more concentration. When the solution is basic, the ions PO43- and HPO42- predominates, while in acid conditions, H2PO4- and H3PO4 predominates.
Uses: Phosphates are used by the biological systems (cells) for forming important molecules as the ATP and ADP that are involved in the metabolism pathway of the respiration. It is also in the DNA and RNA. Phosphates as the sodium and potassium phosphates are widely used as acidity regulators in the food industry.
Health effects/safety hazards: Phosphates are considered safe for the health. They are not toxic and they can be used in pharmaceutical and food industry. Especially, potassium and sodium phosphates are considered the safest and are largely used in medicine.
|
Related Links: |
