Phosphoric acid Formula - Phosphoric Acid Uses, Properties, Structure and Formula
Phosphoric acid is one of the most important and useful mineral acids. It is non-toxic, and is also commonly called orthophosphoric acid.
Formula and structure: The chemical formula of phosphoric acid is H3PO4. Its molecular formula is written as H3O4P and its molar mass is 97.99 g/mol.
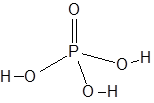
The central phosphorous atom is connected to an oxygen atom through a double bond and to three hydroxyl (-OH) groups through single bonds. Thus, H3PO4 can release up to three H+ ions.
Preparation: Phosphoric acid is produced industrially through a wet process, in which sulfuric acid is reacted with apatite (tricalcium phosphate rock).
Ca5(PO4)3Cl + 5 H2SO4 + 10 H2O → 3 H3PO4 + 5 CaSO4·2 H2O + HCl
The resulting phosphoric acid solution is only about 32-46% H3PO4, so it is then concentrated (by evaporation of water) to produce higher concentration commercial grades of phosphoric acid.
Physical properties: Pure phosphoric acid is a white crystalline solid with melting point of 42.35° C. When it is less concentrated, it is a colorless, odorless, viscous liquid with a density of 1.885 g/mL. It is non-toxic and non-volatile. The most common phosphoric acid concentration is 85% in water.
Chemical properties: Phosphoric acid has three acidic and replaceable H atoms. Thus, it reacts differently from other mineral acids. It can react with bases to form three classes of salts by the replacement of one, two, or three H atoms, such as NaH2PO4, Na2HPO4, and Na3PO4, respectively.
At high temperatures, phosphoric acid molecules can react together and combine (with loss of water molecule) to form dimers, trimers, and even long polymeric chains such as polyphosphoric acids and metaphosphoric acids.
2 H3PO4 → H4P2O7 (- H2O)
Uses: Phosphoric acid has many applications. Due to its non-toxic and mildly acidic nature, it is used in food flavoring, beverages, dental products, cosmetics, and skin care products. Industrially, it is used mainly in the production of phosphate fertilizers. It is also widely used as an electrolyte, etching agent, rust removal agent, pH modifier, household cleaning agent, dispersing agent and sanitizing agent.
Health hazards/ health effects: Phosphoric acid is not considered toxic or hazardous. In low concentrations, it is safe on skin and even for consumption (it is used in food, cosmetics and dental products). However, at very high concentrations, it is corrosive and can cause skin burns.
|
Related Links: |
