Potassium Carbonate Formula
Potassium carbonate, also known as carbonate of potash, dipotassium carbonate or pearl ash, is an inorganic salt used in the production of soap and glass.
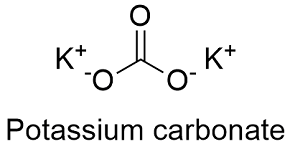
Formula and structure: The potassium carbonate chemical formula is K2CO3 and the molar mass is 138.205 g mol-1. The structure is a central carbonate anion CO32- with two potassium cations attached. It can be a crystal at temperatures below -47 ºC. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: It can be found in caverns together with other carbonates.
Preparation: There are many methodologies to prepare potassium carbonate, some fo them are:
-Through the Engel-Precht process which uses magnesium oxide, potassium chloride and carbon dioxide. This process forms the Engel salt MgCO 3.KHCO3.4H2O which can form pure potassium carbonate after decomposition of the salt.
-By electrolysis of potassium chloride
-By treating potassium hydroxide with excess of carbon dioxide
2 KOH + CO2 → K2CO3 + H2O
Physical properties: Potassium carbonate is a white, hygroscopic solid with and a deliquescent appearance. Its density is 2.43 g mL-1. Potassium carbonate melting is 891 ºC. It is soluble in water. It is insoluble in methanol, ethanol or toluene.
Chemical properties: Potassium carbonate in water forms strong alkali solutions. Carbonate anion that forms the potassium carbonate, is the second anion species originated from the deprotonation of carbonic acid H2CO3.
Uses: Potassium carbonate is used as a fertilizer because it's high content of potassium and for the same reason; it is used to feed animals. It is used in some countries as a bakery powder. It is also used in the preparation of regulators of pH or to prepare buffer solutions.
Health effects / safety hazards: Potassium carbonate is not toxic or flammable but it can cause damage to the health. It is harmful if swallowed and can cause serious eyes and skin irritation.
|
Related Links: |
