Potassium chloride Formula - Potassium chloride Uses, Properties, Structure and Formula
Potassium chloride is an important metal halide which is an essential electrolyte source for the body.
Formula and structure: The chemical formula of potassium chloride is KCl, and its molar mass is 74.55 g/mol. It has a similar crystal structure as sodium chloride (NaCl). Its chemical structure is shown below, in which each KCl molecule consists of one potassium cation (K+) and one chloride anion (Cl-).
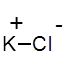
Occurrence: It occurs naturally as the mineral sylvite. It is also found naturally as a mixture with sodium chloride in a mineral called sylvinite. It is also present in seawater.
Preparation: KCl is commonly obtained by mining its minerals, followed by extraction. It is also extracted from brine (salt water). It can also be prepared in the laboratory in small scales by reacting potassium hydroxide (KOH) with hydrochloric acid (HCl).
KOH + HCl → KCl + H2O
Physical properties: KCl is an odorless, white crystalline solid, with a density of 1.98 g/mL, a melting point of 770 °C, and a boiling point of 1420 °C.
Chemical properties: KCl is highly soluble in water and a variety of polar solvents, and insoluble in many organic solvents. KCl dissolves in water and gets fully ionized into solvated K+ and Cl– ions. Thus, aqueous solutions of KCl show electrical conductivity, making KCl an important electrolyte in many applications. Another important reaction of KCl is used to produce metallic potassium, by reducing KCl with metallic sodium at 850 °C.
KCl + Na → NaCl + K
Uses: The main uses of KCl are in electrolytes, pH buffers, and preparation of fertilizers, explosives, potassium metal and potassium hydroxide. Potassium is essential for various functions of the body, and KCl is a key source of this nutrient. It is also used in medicine, food processing, and as a substitute for table salt (sodium chloride). It has several other similar applications as sodium chloride including de-icing roads and homes, in petroleum and natural gas industry, and in water softening.
Health effects/safety hazards: At low concentrations, KCl is non-toxic and essential for the body. However, at high concentrations, potassium chloride is toxic and even lethal. High amounts of KCl can affect the cardiac muscles causing heart attacks and even death.
|
Related Links: |
