Potassium hydroxide Formula - Potassium Hydroxide Uses, Properties, Structure and Formula
Potassium hydroxide is an important inorganic base, and is also called caustic potash or potash lye.
Formula and structure: The chemical formula of potassium hydroxide is KOH, and its molar mass is 56.11 g/mol. The structure of KOH consists of an ionic bond between the potassium metal cation and the hydroxyl anion, as shown below. Solid KOH is found in a rhombohedral crystalline structure, similar to that of sodium chloride.
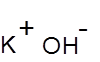
Preparation: The industrial preparation of KOH is similar to that of NaOH, by the chloralkali process. It is prepared by the electrolysis of potassium chloride solutions, along with chlorine gas as a by-product:
2 KCl + 2 H2O → 2 KOH + Cl2 + H2
Physical properties: Potassium hydroxide is a white solid with a density of 2.12 g/mL, a melting point of 360 °C, and boiling point of 1,327 °C. It is typically available as translucent pellets, or as aqueous solutions of different concentrations.
Chemical properties: KOH is a highly hygroscopic solid which absorbs water from air, thus making it a useful laboratory desiccant (drying agent). It is very stable thermally (does not decompose even at high temperatures). It dissolves in water to form strongly alkaline, aqueous KOH solutions called potassium lye. It readily reacts with acids to form a variety of potassium salts, which have many uses in industry.
Uses: Potassium hydroxide is used in many of the same applications as sodium hydroxide. In addition, aqueous KOH is used as the electrolyte in alkaline batteries. It is also used to manufacture soft soaps and liquid soaps by saponification. Many industrially useful potassium salts are prepared by reaction with KOH. Some of the other uses of KOH are in chemical manufacturing, fertilizer production, petroleum refining, and cleaning solutions.
Health effects/safety hazards: KOH is a strong and corrosive base, which can penetrate skin and tissues. Skin or eye contact with KOH solutions can cause burns, severe irritation, and even blindness. Inhalation of this corrosive base can damage the mucous membranes and lungs. If swallowed, it is extremely toxic and can lead to permanent tissue damage and be fatal.
|
Related Links: |
