Potassium Nitrate Formula
Potassium nitrate, commonly named as saltpeter or nitrate of potash, is a inorganic salt that has been used since Ancient age as fertilizer and medicines.
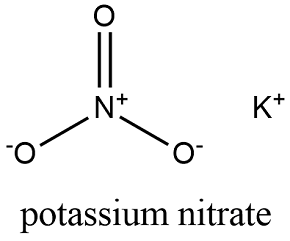
Formula and structure: The potassium nitrate chemical formula is KNO3 and the molar mass is 101.102 g mol-1. This salt is formed by one potassium cation (K+) and one nitrate anion (NO3-). These species form an ionic salt. The crystal structure is orthorhombic but at high temperatures (above 130 °C), it has a trigonal structure. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Potassium nitrate is found in some minerals such as niter. It is also found in caves, particularly due to the presence of bat guano, which is a natural source of potassium nitrate.
Preparation: Potassium nitrate is produced from the reaction of potassium chloride with nitric acid at high temperatures. This acid can also react with carbonate of potash, resulting in potassium nitrate as well.
HNO3 (aq) + KCl (aq) → HCl (aq) + KNO3 (aq)
Physical properties: Potassium chloride is a white to light-gray, odorless solid. Its density is 2.109 g mL-1.The melting and boiling points are 334°C and 400°C. It is soluble in water and its solubility increases with the temperature. It is insoluble in alcohol.
Chemical properties: Potassium nitrate can be used to prepare neutral solutions since the pH of its solutions in water reach a value of 6.2, so it can be used as an additive in food and preservatives. In this solution, potassium nitrate reaches equilibrium with potassium nitrite.
2 KNO3 ⇌ 2 KNO2 + O2
Uses: Potassium nitrite is used to making gunpowder. It is also a raw material in the preparation of toothpaste, rocket propellants and fireworks. Due to the presence of nitrate anion, it is a good fertilizer. Potassium nitrate is a also used as a diuretic agent in pigs, cattle and horses.
Health effects / safety hazards: Potassium nitrate is non flammable but in presence of combustible materials, it can burn or explode. It can intensify fire. Potassium nitrate causes skin and eyes irritation.
|
Related Links: |
