Pyrosulfuric acid Formula - Pyrosulfuric Acid Uses, Properties, Structure and Formula
Pyrosulfuric acid is a strong acid which is a main constituent of 'fuming' sulfuric acid (oleum). It is also commonly known as disulfuric acid.
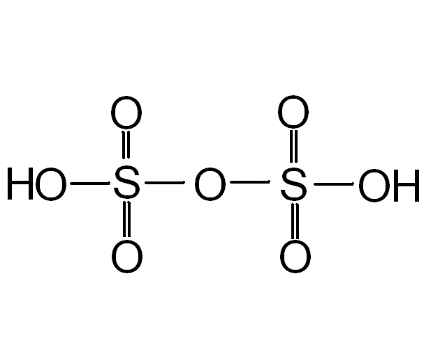
Formula and structure: The chemical formula of pyrosulfuric acid is H2O7S2 and its molar mass is 178.13 g/mol. The chemical structure is shown below. Pyrosulfuric acid can be considered as an anhydride of sulfuric acid (H2SO4), since its structure is the same as one would get by combining two molecules of sulfuric acid, followed by dehydration of one molecule of water.
Preparation: Pyrosulfuric acid is prepared by dissolving sulfur trioxide (SO3) in concentrated sulfuric acid:
H2SO4 + SO3 → H2S2O7
It is also obtained as an intermediate during the preparation of sulfuric acid, through the contact process.
Physical properties: Pure pyrosulfuric acid is a colorless crystalline solid with a melting-point of 36° C. It is more commonly found in a less pure form as a fuming, dense, oily liquid which can be colorless to dark brown depending on its purity level. Since it is usually found as a mixture with some amounts of sulfuric acid, its physical properties are variable.
Chemical properties: The chemical properties of pyrosulfuric acid resemble those of sulfuric acid. However, it is a much weaker acid than sulfuric acid. It readily reacts with bases to form salts called pyrosulfates, such as sodium and potassium pyrosulfates. Pyrosulfuric acid decomposes on heating to give a mixture of sulfuric acid and water, after elimination of sulfur trioxide.
Uses: Its main uses are in the manufacture of explosives and dyes. It is also used widely as a sulfating agent and in petroleum refining. It is also commonly used as a convenient intermediate or form of sulfuric acid for storage and transportation, due to its less corrosive nature. It can be diluted in water to readily give sulfuric acid of the desired concentration.
Health hazards/ health effects: Its health effects are similar to those of sulfuric acid. Pyrosulfuric acid is a corrosive acid and a strong dehydrating agent. It can cause severe burns on skin contact, and exposure to the fumes can cause severe irritation to the eyes, mucous membranes and respiratory tract. Swallowing can lead to permanent tissue damage and can be fatal.
|
Related Links: |
