Silver Perchlorate Formula
Silver perchlorate is an inorganic silver compound that is known for its explosiveness.
Formula and structure: The chemical formula of silver perchlorate is AgClO4 and its molar mass is 207.319 g/mol. It is a salt composed of the silver cation (Ag+) and the perchlorate anion (ClO4-), in which the central chlorine atom is covalently bonded to three oxygen atoms through double bonds and one oxygen atom through a single bond, as shown below.
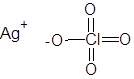
Preparation: Silver perchlorate is commonly prepared by reacting a mixture of perchloric acid (HClO4) with silver nitrate (AgNO3) at high temperatures. It is also prepared through other routes such as the reaction between barium perchlorate and silver sulfate, or the reaction between perchloric acid and silver oxide.
AgNO3 + HClO4 → AgClO4 + HNO3
Physical properties: Silver perchlorate is a white solid with a density of 2.81 g/mL and melting point of 486 °C. It commonly exists as a monohydrate (AgClO4.H2O) and is mildly deliquescent (absorbs moisture from air and turns into liquid).
Chemical properties: Silver perchlorate is highly soluble in water (unlike many other silver salts) as well as in several organic aromatic solvents. It is highly reactive and readily forms explosive mixtures. It reacts explosively with several chemicals including acetic acid, aniline, diethyl ether, diaminoethane, aromatic hydrocarbons, etc. It is self-reactive and its crystals can explode upon crushing. It is stable at room temperature in inert atmosphere, but when heated to high temperatures, it decomposes with the release of toxic fumes of chlorine.
Uses: Silver perchlorate is used in the manufacture of various explosives and as a catalyst in organic synthesis. Despite its explosive nature, AgClO4 is widely used in chemical synthesis as it is a useful source of the silver ion and a precursor for many silver compounds.
Health effects/safety hazards: Silver perchlorate is a hazardous and explosive chemical and must be handled with care. It can cause chemical burns when exposed to skin or eyes. Inhalation of the solid can cause respiratory irritation, while swallowing it can cause nausea, vomiting and chemical burns within the mouth and digestive tract.
|
Related Links: |
Related Topics
Chemistry Formulas
Formulas: Physics Formulas and Math Formulas
General Chemistry topics
Ionic and Net Ionic Equations
