Sodium amide Formula - Sodium amide Uses, Properties, Structure and Formula
Sodium amide is a strong inorganic base, which is also called sodamide.
Formula and structure: The chemical formula of sodium amide is NaNH2, and its molar mass is 39.01 g/mol. NaNH2 is a salt-like material which has a tetrahedral crystal structure. It is composed of sodium ions (Na+) and amide (NH2-) ions, and its chemical structure is shown below. The free amide ions make this molecule a strong base.
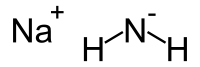
Preparation: Sodium amide is prepared by reacting sodium metal with ammonia gas or liquid ammonia. The common production method uses sodium in liquid ammonia with iron nitrate catalyst to speed up the process. The reaction proceeds with the formation of an electride intermediate, which then quickly gives sodium amide and hydrogen gas.
2 Na + 2 NH3 → 2 NaNH2 + H2
Physical properties: Sodium amide exists as colorless crystals with a strong ammonia odor. Its density is 1.39 g/mL, melting point is 210 °C and boiling point is 400 °C.
Chemical properties: Sodium amide is highly reactive and reacts violently with water to produce ammonia gas and caustic sodium hydroxide.
NaNH2 + H2O → NH3 + NaOH
In closed containers, it rapidly absorbs H2O and CO2 from the air to form peroxides, which are explosive compounds. For this reason, sodium amide is stored under inert gases (such as argon or nitrogen) in tightly sealed containers. NaNH2 dissolves in liquid ammonia to give an ionic conductive solution. It is insoluble in many organic solvents.
Uses: Sodium amide is a strong base and used in many chemical reactions for this purpose, especially in organic synthesis. It is also used in the preparation of some dyes (such as indigo) and several important organic compounds (such as hydrazine, sodium cyanide).
Health effects/safety hazards: Sodium amide reacts violently with air and water, and also forms explosive peroxides. It is also a serious health hazard. On inhalation, it can cause severe irritation or even chemical burns to the eyes, mucous membranes and respiratory tract. Inhalation or ingestion can lead to severe gastrointestinal burns and be fatal.
|
Related Links: |
