Sodium Bisulfate Formula - Uses, Properties, Structure and Formula
Sodium bisulfate is an acidic salt of sulfuric acid and is also known as sodium hydrogen sulfate.
Formula and structure: The chemical formula of sodium bisulfate is NaHSO4 and its molar mass is 120.06 g/mol. It also exists as a monohydrate salt (with one molecule of water) with the molar mass of 138.07 g/mol. It is the monosodium salt of the diprotic sulfuric acid. It is composed of the sodium cation (Na+) and bisulfate anion (HSO4-), which has the sulfur atom bonded to three oxygen atoms and one hydroxyl group.
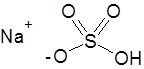
Preparation: Sodium bisulfate can be prepared industrially by the partial neutralization of sulfuric acid with equimolar amounts (1:1 ratio) of sodium hydroxide (NaOH) or sodium chloride (NaCl):
NaOH + H2SO4 → NaHSO4 + H2O
Sodium bisulfate is also obtained as a byproduct during the production of other mineral acids such as nitric acid (HNO3) and hydrocyanic acid (HCN).
NaNO3 + H2SO4 → NaHSO4 + HNO3
Physical properties: Sodium bisulfate is commonly available in both anhydrous (dry) and monohydrate (NaHSO4.H2O) forms. Anhydrous NaHSO4 is an amorphous and hygroscopic white powder, with density of 2.74 g/mL and melting point of 315 °C. The monohydrate is a white granular solid with a density of 1.8 g/mL and a melting point of 59 °C. It is considered a dry acid suitable for safe shipping and storage.
Chemical properties: Sodium bisulfate is highly water soluble. It is chemically an acidic salt, rather than a typical neutral salt, due to substitution of only one acidic proton of the diprotic sulfuric acid. Aqueous solutions of NaHSO4 are highly acidic. It reacts violently with strong bases and strong oxidizing agents. It is stable under normal conditions, but decomposes upon exposure to water.
Uses: Sodium bisulfate is commonly used as a preservative, food additive, in meat processing, in dietary supplements and in drugs. It is used in pH control, disinfecting, household cleaning products, metal cleaning, leather tanning, swimming pools, and many other applications.
Health effects/safety hazards: Sodium bisulfate is very irritating to the eyes and skin due to its strong acidic nature. At high concentrations, it can cause serious eye damage upon contact. It can cause severe diarrhea and vomiting if swallowed, and is very toxic in large amounts.
|
Related Links: |
Related Topics
Chemistry Formulas
Formulas: Physics Formulas and Math Formulas
General Chemistry topics
Ionic and Net Ionic Equations
