Sodium Bromide Formula
Sodium bromide is the most used bromide in the chemical industry. It is mainly used as a source of bromide ion and as catalyst.
Formula and structure: Sodium bromide chemical formula is NaBr and the molar mass is 102.89 g mol-1. It can be found hydrated by one (monohydrate NaBr) or two (duhydrate NaBr) water molecules. Sodium bromide is formed by one sodium cation Na+ and one bromide anion Br- which are joined through an ionic bond. The crystal structure has the form of a face-centered cubic with six bromide anions surrounded one sodium cation. Its chemical structure can be written as below, in the common representations used for organic molecules.
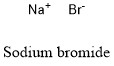
Occurrence: Sodium bromide is not found in nature. It is prepared as described below.
Preparation: Sodium bromide is mostly prepared from the reaction of sodium hydroxide with hydrogen bromide:
NaOH + HBr → NaBr + H2O
Physical properties: Sodium bromide is a white, hygroscopic solid. Its density is 2.18 g mL-1. The melting point of this salt is 747 °C, while the boiling point is 1390 °C. Sodium bromide is very soluble in water, liquid ammonia, pyridine and it is insoluble in acetone and acetonitrila.
Chemical properties: Sodium bromide is mainly used in the preparation of other bromides, such as potassium bromide and the reason is the bromide ion Br- is a strong nucleophile that can attack alkyl chloride and through a nucleophilic substitution reaction forms the corresponding bromide:
NaBr + RCl → RBr + NaCl (R is na alkyl group, for example CH3)
Sodium bromide can also be a source of the element bromine, which can be produced by the reaction of sodium bromide with chlorine gas:
2 NaBr + Cl2 → Br2 + 2 NaCl
Uses: Sodium bromide was used as medicine for around 70 years, as an anticonvulsant and sedative, however it is not longer used. As catalyst, it is used to promote some reactions in organic synthesis and it is also largely used as the precursor and main reagent in the production of other bromides. Sodium bromide is also a disinfectant.
Health effects / safety hazards: Sodium bromide is not toxic at low concentration. However, it is enough toxic in medium to high concentration to be considered unsafe to use as medicament. It is not flammable and does not react with other substances.
|
Related Links: |
