Sodium carbonate Formula - Sodium carbonate Uses, Properties, Structure and Formula
Sodium carbonate is an inorganic compound used as a moderate strength base. It is also called soda ash or washing soda.
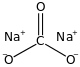
Formula and structure: The chemical structure of sodium carbonate is Na2CO3, and its molar mass is 106.0 g/mol. Sodium carbonate is an ionic compound- a sodium salt of carbonic acid, and is composed of two sodium cations (Na+) and a carbonate anion (CO3-). It has a crystalline heptahydrate structure.
Occurrence: Sodium carbonate occurs naturally in mineral form as its hydrate salts (such as trona, natron, natrite, etc.). There are several of its mineral deposits found in dry regions around the world.
Preparation: Sodium carbonate is obtained commercially through two different methods. The first method involves mining the mineral deposits of sodium carbonate, which is the main production method in the USA. In the second method, called the Solvay process, sodium chloride is reacted with ammonia to give sodium bicarbonate, which is then heated to give sodium carbonate.
Physical properties: Sodium carbonate is a white crystalline powder with a density of 2.54 g/mL, and a melting point of 851 °C.
Chemical properties: Sodium carbonate is a stable but hygroscopic solid (absorbs water from air) and readily dissolves in water to form weakly acidic carbonic acid and the strong base, sodium hydroxide. Thus, the aqueous solution of Na2CO3 is overall a strong base. It reacts violently with many acids. When heated to high temperatures, it decomposes to emit toxic fumes of disodium oxide (Na2O).
Uses: The main uses of sodium carbonate are as water softener, food processing aid, pH modifier, swimming pool chemical and electrolyte. It is also used in the manufacture of glass, paper, soaps and detergents, and many other useful chemicals.
Health effects/safety hazards: Sodium carbonate solutions are strongly alkaline and corrosive. It can cause severe skin and eye irritation upon contact. Inhalation of sodium carbonate dust or fumes can cause irritation of mucous membranes and the respiratory tract, and lead to severe coughing and shortness of breath. High concentrations can damage the eye and cause skin burns.
|
Related Links: |
