Sodium Chromate Formula - Sodium Chromate Uses, Properties, Structure and Formula
Sodium chromate, also called sodium chromate (VI), is a brightly colored inorganic salt used for various applications.
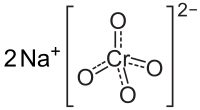
Formula and structure: Sodium chromate has the chemical formula Na2CrO4, and a molar mass of 161.97 g/mol. It is a salt made of two sodium cations (Na+) and the chromate anion (CrO4-) in which the chromium atom is attached to four oxygen atoms. The oxidation state of chromium metal is +6 in the chromate salt. The solid material exists in an orthorhombic crystalline structure.
Preparation: It is industrially prepared by heating chromium ores such as chromite (Fe(CrO2)2) in air along with sodium carbonate. The water soluble sodium chromate is easily separated from the iron oxide.
4 Fe(CrO2)2 + 8 Na2CO3 + 7 O2 → 8 Na2CrO4 + 2 Fe2O3 + 8 CO2
Physical properties: Sodium chromate is a yellow, crystalline solid which is hygroscopic (absorbs water from air). It is an odorless solid with a density of 2.7 g/mL, and a melting point of 792 °C. It also exists in tetrahydrate (Na2CrO4.4H2O), hexahydrate (Na2CrO4.6H 2O), and decahydrate (Na2CrO4.10H2O) forms.
Chemical properties: The hygroscopic sodium chromate solid readily absorbs water and turns into its hydrated salts. It dissolves in water to give yellow chromate ions. Sodium chromate is a moderate oxidizing agent. It readily reacts with acids to give the important industrial chemical, sodium dichromate:
2 Na2CrO4 + H2SO4 → Na2SO4 + Na2Cr2O7 + H2O
Uses: The major applications of sodium chromate are to produce sodium dichromate (an important precursor to most chromium compounds) and the extraction of chromium metal from its ores. Its other applications include corrosion control, pigment formulations, wool dyeing, wood preservation, drilling fluids, catalysts and as oxidant in organic synthesis.
Health effects/safety hazards: As a hexavalent chromium chemical, sodium chromate is highly toxic and carcinogenic (causes cancer). It is also corrosive and causes a burning sensation in eyes and skin. It is very harmful when swallowed and causes diarrhea, shock and liver damage. When heated, it decomposes to give toxic chromium oxide fumes, which are also very irritating to eyes, skin and mucous membranes.
|
Related Links: |
