Sodium cyanide Formula - Sodium cyanide Uses, Properties, Structure and Formula
Sodium cyanide is a highly poisonous inorganic compound, which is widely used in the chemical and mining industries.
Formula and structure: The chemical formula of sodium cyanide is NaCN, and its molar mass is 49.01 g/mol. It is an ionic compound composed of the sodium cation (Na+) and cyanide anion (CN-), in which the carbon has a triple bond with the nitrogen atom. Solid NaCN adopts a crystalline structure similar to that of sodium chloride (NaCl).
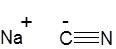
Preparation: Sodium cyanide is industrially prepared by the reaction of hydrogen cyanide with sodium hydroxide:
HCN + NaOH → NaCN + H2O
Physical properties: Sodium cyanide is a white crystalline solid with a faint odor of almonds. It has a density of 1.59 g/mL, and a melting point of 564 °C. The NaCN crystals are highly water soluble and deliquescent, meaning that they can absorb a lot of water from the atmosphere and turn into liquid.
Chemical properties: Sodium cyanide is the salt of a weak acid (hydrogen cyanide, HCN), and gets easily hydrolyzed by water to form the highly toxic HCN gas. Even the solid NaCN crystals can absorb water from the atmosphere and start releasing hydrogen cyanide gas, making it highly dangerous. It also readily reacts with acids to release HCN gas:
NaCN + H2SO4 → HCN + NaHSO4
Sodium cyanide can be rendered safe or detoxified by treating it with hydrogen peroxide (H2O2), which gives the much less toxic sodium cyanate (NaOCN) as the product.
NaCN + H2O2 → NaOCN + H2O
Uses: The main application of this toxic compound is in the mining industry, where it is used to extract gold and other precious metals from their ores. It is also used for electroplating. It is used as an important precursor to many useful organic and inorganic chemicals, including pharmaceuticals. Due to its toxicity, it is used as an insecticide to kill insects and pests. Another important use of NaCN is in analytical testing.
Health effects/safety hazards: Sodium cyanide is extremely poisonous due to its being a cyanide salt. It is one of the fastest acting poisons and is fatal even if swallowed in small amounts. Exposure to the solid NaCN can also be dangerous due to its ability to release the poisonous HCN gas slowly in air.
|
Related Links: |
