Sodium iodide Formula - Sodium Iodide Uses, Properties, Structure and Formula
Sodium iodide is a common inorganic salt that is an important source of iodine.
Formula and structure: The chemical formula of sodium iodide is NaI and its molar mass is 149.89 g/mol. It is a simple ionic compound composed of the sodium cation (Na+) and iodide anion (I-), and its chemical structure is shown below. The solid NaI adopts a similar octahedral crystal geometry as sodium chloride (NaCl).
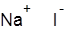
Preparation: Sodium iodide is produced industrially by the reaction between sodium hydroxide and iodine or hydroiodic acid. It is also prepared by treating sodium carbonate with hydroiodic acid solution.
NaOH + HI → NaI + H2O
Physical properties: It is found as white, odorless crystals or powder with density of 3.67 g/mL, melting point of 651 °C and boiling point of 1,304 °C. It is a deliquescent solid, which absorbs moisture and turns into solution.
Chemical properties: Sodium iodide is very soluble in water and some organic solvents. It is sensitive to air, moisture and light, and solid NaI turns brown on exposure to air or light, due to formation of iodine fumes. It also reacts violently with strong oxidants, strong acids and bromine trihalides, producing iodine.
Uses: Sodium iodide is commonly used as a dietary iodine supplement and included in table salt (NaCl). It is used to treat and prevent iodine deficiency. Another common application of NaI is in nuclear medicine, as radioactive sodium iodide (such as NaI125 and NaI131) and its derivatives are important radiopharmaceuticals that are used for treating thyroid cancer and hyperthyroidism, and also as imaging tracers. Sodium iodide is also used as a reagent in organic synthesis to prepare various alkyl iodides.
Health effects/safety hazards: Sodium iodide is not toxic in recommended dosages and is used as a dietary supplement. However, large doses can cause a condition called iodism, which results in headache, fever, excess salivation, bronchitis, diarrhea and vomiting. Inhalation of the sodium iodide dust can cause irritation of throat and mucous membranes. Also, due to its air and light sensitivity, solid NaI easily emits iodine fumes which are very irritating and corrosive.
|
Related Links: |
