Sodium thiosulfate Formula
Sodium tiosulfate, is an inorganic salt widely used in the pharmaceutical industry and also as drugs. It is included in the list of Essential Medicines of the WHO (World Health Organization).
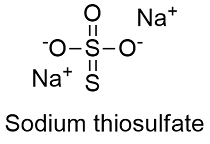
Formula and structure: The sodium thiosulfate chemical formula is Na2S2O3 and its molar mass is 158.11 g mol-1. Sodium thiosulfate mostly exists as a pentahydrated salt Na2S2O3.H2O and its molar mass is 248.18 g mol-1. The molecule is formed by the thiosulfate anion S2O3-2 and the sodium cation Na+1. The thiosulfate anion has a tetrahedral geometry; it is basically a sulfate anion SO4-2 where an oxygen atom has been replaced by a sulfur atom, and the sodium atoms are placed between the S-O bonds. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Sodium thiosulfate and the other thiosulfate are not found in nature. These compounds are produced by chemical synthesis.
Preparation: Sodium thiosulfate can be prepared through several methods: for example from liquor formed in the production of the sodium sulfite is reacted with sulfur dioxide and to yield the Na2S2O3.
SO2 + Na2S → Na2S2O3
Other method is the boiled of nitro compounds with sodium polysulfide solution or by dissolving sulfur in sodium sulfite.
Physical properties: Sodium thiosulfate is an odorless, white crystalline solid. Its density is 1.667 g mL-1. The melting and boiling points of the pentahydrate salt are 48.3 ºC and 100 ºC, respectively. In higher temperatures and in acids solutions, it decomposes. Sodium thiosulfate is very soluble in water, but is insoluble in ethanol.
Chemical properties: Sodium thiosulfate is very versatile to use in chemical processes due it can undergo many types of different reaction. It is unstable in the presence of acids because the molecule is highly susceptible to suffer protonation in the most terminal sulfur atom yielding to sulfur dioxide, water and sulfur:
Na2S2O3 + 2 HCl → 2 NaCl + S + SO2 + H2O
Sodium thiosulfaye is also a reducing agent used to reduces chlorines and iodines in organic synthesis:
Na2S2O3 + 4 Cl2 + 5 H2O → Na2SO4 + H2SO4 + 8HCl
Na2S2O3 + I2 → Na2S4O6 + 2 Nal
Sodium thiosulfate can also be used to form complex with certain metal ions, such as silver:
AgBr + 2 Na2S2O3 → Na3[Ag (S2O3)2] + NaBr.
Uses: Sodium thiosulfate is mainly used by the pharmaceutical industry as a drug: it is used in different treatments such as hemodialysis or chemotherapy. Its characteristic as sulfur donor is used in antidote against cyanide poisoning because it allows th conversion of cyanide to thiocyanide. Sodium thiosulfate is also an antifungal agent. Other application is as photographic fixer adding to the emulsions to reveal photos, in purification of wasters form gold mining or chlorinated water.
Health effects / safety hazards: Sodium thiosulfate can cause skin irritation. It can also cause severe eye and respiratory system irritation. It is not flammable, however can react with acids to decomposition.
|
Related Links: |
