Specific Heat Capacity Formula
The specific heat capacity of a substance is the amount of heat required to raise one gram of the substance by one degree Celsius. Water, for example, has a specific heat capacity of 4.18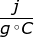 . This means to heat one gram of water by one degree Celsius, it would require 4.18 joules of energy.
. This means to heat one gram of water by one degree Celsius, it would require 4.18 joules of energy.
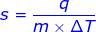
s = specific heat capacity (sometimes represented by the letter c, or Cs)
q = heat
m = mass
Δ T = change in temperature
Specific Heat Capacity Formula Questions:
1. What is the specific heat capacity of iron if it takes 125 J of heat to raise 111 grams by 2.5 degrees Celsius?
Answer:
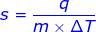
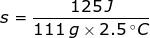
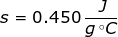
2. What is the specific heat capacity of aluminum if it takes 2500 J to raise 150 grams from 10°C to 28.5°C?
Answer:
In this problem the change in temperature must be determined by taking the final temperature (Tf) minus the initial temperature (Ti).
Δ T = Tf - Ti
Δ T = 28.5°C – 10°C
Δ T = 18.5°C
Continue by solving the equation for specific heat capacity.
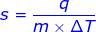
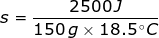
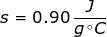
|
Related Links: Heat Transfer Formula Specific Heat Formula |
