Strontium fluoride Formula
Strontium fluoride, also known as strontium difluoride, is an inorganic and binary salt used to manufacture optical materials for different devices.
Formula and structure: The strontium fluoride chemical formula is SrF2 and its molar mass is 125.62 g mol-1. The molecule is formed by one cation strontium Sr+2 and two anions fluoride F-1 and the structure is highly similar to other fluoride formed by a cation of the second group of the periodic table: calcium fluoride, which has a cubic crystal system. Its chemical structure can be written as below, in the common representations used for organic molecules.
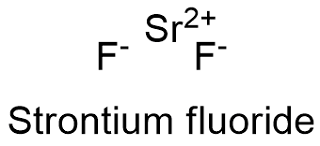
Occurrence: Strontium fluoride is not found in nature, it is just produced by chemical processes.
Preparation: Strontium fluoride is produced by a process known as electrosynthesis, which applies electrochemical techniques to promote the reaction between fluorine gas or hydrofluoric acid and the salts strontium chloride or carbonate:
SrCl2 + F2 → SrF2
Physical properties: Strontium fluoride is colorless or white powder. Its density is 4.24 g mL-1 and the melting and boiling points are 1400 ºC and 2489 ºC. It is poorly soluble in water, ethanol and methanol.
Chemical properties: Strontium fluoride is a molecule formed by the fluoride anions, which is the most electronegative element of the periodic table (it means that has more capacity to attract electrons in a molecule), thus it is waited the electrons on the strontium fluoride molecule are most of the time under the fluoride atoms and create very polarizable bounds, modifying the structure to form a tetrahedral geometry and it also gives an elevate ionic conductivity to this salt.
Uses: Strontium fluoride is very used to manufacture of optical material to produce many devices due to its high conductivity and its optical properties similar to calcium fluoride. Strontium fluoride has the capacity of transmit ultraviolet and infrared waves, thus it is used in glasses, windows and lenses.
Health effects / safety hazards: Strontium fluoride is very danger to the health, it is poisonous when inhaled or ingested and in low concentration it can cause irritation to skin and eyes. It is not flammable.
|
Related Links: |
