Sulfate Formula
Sulfate, also known as sulfate ion or sulphate, is a polyatomic anion that is part of many salts largely used as chemical compounds or as a raw material in industry.
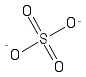
Formula and structure: The sulfate chemical formula is SO42-. The molar mass is 96.06 g/mol. The molecule is formed by one centered sulfur S6+ and four anions O2-. Two oxygen atoms are bound with double bonds to the sulphur atom. The other two atoms are bound through single bonds and one negative charge is over each of these oxygen atoms, forming a total charge of -2. The geometry of the molecule is tetrahedral. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Sulfate ion is found in all the organisms. Particularly, micro-organisms living in extreme conditions as the ones living in thermal vents, have special enzymes to produce energy through redox reactions between sulfate and other organic compounds.
Preparation: Although sulfate can be found in nature and can be extracted from the oxidation of metal sulfides, there are some chemical methods to prepare it. Some of these reactions use metals or metallic hydroxides along with sulfuric acid:
Zn + H2SO4 → ZnSO4 + H2
Cu(OH)2 + H2SO4 → CuSO4 + 2 H2O
CdCO3 + H2SO4 → CdSO4 + H2O + CO2
Physical properties: Sulfate ions physical properties in general depends on the cation that is bound to it, forming the salt. Many of these salts are solids and are highly soluble in water. The exception are the salts formed by the cations of the group 2 of the periodic table: calcium, strontium, barium, radium and also lead, that form insoluble salts with the sulfate anion.
Chemical properties: Sulfate ion is highly stable and in part this stability is caused by the resonance effect on its structure. Although there are two double bonds and two single bonds between the oxygen atoms and the sulfur atom, the fact is all the bonds are equivalent and have the same distance and charge. In chemistry, it is due to the resonance effect that cause a fast-movement exchanging the two configuration of sigma and pi bond, making that all the bonds have a structure that is in the average of the two types of bonds. It creates stable structures in the molecules.
Uses: Sulfates are largely used in chemical industry, chemical synthesis as a isolated compounds in the form of salts. Some examples include the mineral formed by calcium sulfide, barium sulfate, copper sulfate, magnesium sulfate and others. Sulfates are also used as fertilizer and in food and cosmetics for preservation.
Health effects / safety hazards: Sulfate health effects depend on the salt formed. In general, sulfate salts are not flammable and safety for health.
|
Related Links: |
