Toluene Formula
Toluene, also known bu the IUPAC name of methylbenzene or toluol, is an important solvent used in chemical and pharmaceutical industries and also in many laboratories around the world.
Formula and structure: The toluene chemical formula is C7H8 and its molar mass is 92.14 g mol-1. The molecule is formed by phenyl ring (from benzene) and 1 methyl group as substituent. The molecule is planar due to the requirement of sp2 hybridization carbon to promote the aromatic effect of the ring. Its chemical structure can be written as below, in the common representations used for organic molecules.
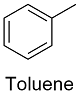
Occurrence: Toluene can be found in nature mostly in Tolú´s balsam where it is extracted from distillation to remove the other component of the resin. It can also be found in lime oil and in crude oil.
Preparation: Toluene can be prepared through organic syntheses or extracted from natural sources. It is prepared through an alkylation of benzene, using methanol:
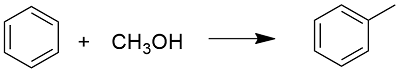
It can also be obtaining from the cyclization of n-heptane followed by an aromatization. But, most of the toluene is extracted from crude petroleum oil, using the distillation, representing the 87% of the total production.
Physical properties: Toluene is a colorless liquid with pungent odor. Its density is 0.87 g mL-1. The melting point is -95 ºC and the boiling point is 111 ºC. It is less dense than water and is insoluble in water
Chemical properties: Toluene is a second most extended benzene-like aromatic compound and similar to this, it can suffer electrophilic aromatic substitution and it is particularly interesting because the substitution of a methyl group in toluene, made it more reactive than benzene, which can suffer sulfonation or chlorination and also oxidation of the methyl group to form a carboxylic acid.
Uses: Toluene is mostly used as solvent in industries and laboratories. Moreover, it is used as a precursor of reaction of substitution and oxidation to perform ready-to-use products or other precursor. It is also added to the gasoline to improve the octane ratings and is an important combustible in aviation. Toluene is very used to produce paints, coats and resins.
Health effects / safety hazards: Toluene is an irritator to eyes. It can be toxic by inhalation in large quantities and also by ingestion. It is a potential carcinogen agent. It is flammable
|
Related Links: |
