Triethylamine Formula
Triethylamine (also know as TEA) is an organic base used in organic synthesis. It is largely used in esters and amides synthesis from acyl chlorides.
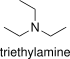
Formula and structure: The chemical and molecular formula of triethylamine are N(CH2CH3)3 and C6H15N, respectively. It is also represented as Et3N and its molecular mass is 101.19 g mol-1. Triethylamine is a tertiary amine consisting in three ethyl groups (-CH2CH3) linked to a nitrogen atom (N). Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Triethylamine is not easily found in nature. However, it is present in some living organism as xenobiotic compounds. These organisms catalyze the excretion trough monoamine oxidase enzymes, through deamination processes.
Preparation: Triethylamine can be prepared by various methods. However, the most extended method is the alkylation of ammonia with ethanol, in the presence of hydrogen and Cu-Ni catalyst:
NH3 + 3 C2H5OH → N(C2H5)3 + 3 H2O
Ethylamine and diethylamine are secondary products of this reaction. Triethylamine can also be synthesized from acetaldehyde, ammonia and hydrogen, through hydrogenation catalyst.
Physical properties: It is a colorless volatile liquid with a fishy and ammoniacal odor. Its density is 0.726 g mL-1 and its boiling point is 88.8 ºC. It is slightly soluble in water at 20 ºC. It is soluble in ethanol, carbon tetrachloride and ethyl ether and very soluble in acetone, benzene and chloroform.
Chemical properties: Triethylamine is an aliphatic amine considered a weak base (pKah is 10.75). Triethylamine is widely used in organic syntheses due its the most simple tri-substituted uniformly amines liquid (trimethylamine is a colorless gas at room temperature). Triethylamine emits toxic vapors of nitrogen oxides when heated.
Uses: Triethylamine is used as organic base, removing the hydrogen from the secondary amine, in the synthesis of esters and amides from acyl chlorides. The reaction products include a quaternary ammonium salt:
R2NH + R'C(O)Cl + Et3N → R'C(O)NR2 + Et3NH+Cl-
Triethylamine is use to manufacture a great variety of chemical compounds like fuels, aditives, intermediats, preservatives, surfactants and fungicides.
Health effects/safety hazards: Triethylamine is extremely flammable and corrosive. Moreover, it forms explosive mixtures with air. Triethylamine can burn skin, eyes and respiratory system. It is incompatible with strong oxidizers, strong acids and halogenated compounds.
|
Related Links: |
Related Topics
Titration Formula
