Urea Formula
Urea, also known as carbamide, is a chemical compound found in the metabolism of nitrogen in animals. It is largely used as fertilizer and raw material to produce other chemical substances.
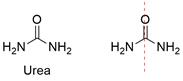
Formula and structure: Urea chemical formula is CO(NH2)2. Its molar mass is 60.06 g mol-1. The molecule is formed by two amide groups (-NH2) bound to a central carboxylic carbon (-C=O), thus urea is a double amide. Consequently, the molecule has a planar geometry in the center (caused by carboxylic carbon sp2) but the amide groups are sp3 allowing the free rotation of bounds. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Urea is formed as the final products of protein metabolism of animals and humans. It is the main compound present in the urine of humans and it is formed through the urea cycle. Urea was discover in 1727 by the dutch chemist Herman Boerhaave. It was first synthesized in laboratorio in 1828 by the german chemist Friedrich Wohler.
Preparation: Urea is obtained by several processes. One of methods include a reaction of ammonia, carbon monoxide and sulfur in methanol. This reaction has two steps, the first one incluede the formation of tone amide group while the second step results in the final compound. It is prepared from liquid ammonia and liquid carbon dioxide and it is performed at high pressure and temperatures.
2NH3+ CO2 ⇌ H2N-COONH4
H2N-COONH4 ⇌ (NH2)2CO + H2O
Physical properties: Urea is a colorless to white, odorless crystalline solid or pellets. Urea density is 1.335 g mL-1. Its melting point is 134 ºC and in higher temperatures urea decomposes. It is very soluble in water (105 kg of urea can be dissolved in 100 kg water), ethanol and methanol. It is insoluble in ether, benzene and chloroform.
Chemical properties: Urea can absorb water from moisturized environments, thus it is used as a dry powder. Other interesting chemical behavior is when it is dissolved in hot water, hydrolyzing into carbamate, ammonia and carbon dioxide. Additional, urea can react with alcohols and esters to form urethanes and barbituric acids.
Uses: Urea is widely use as fertilizer due its large quantity of nitrogen. It is also used as a raw material of animal feed, pesticides, herbicides, fungicides and compounds derived of urea, such as urea-formaldehyde and urea-melamine-formaldehyde. Urea is adding to animal feed. It is used in the explosive industry to make urea nitrate, which is a stabilizer in nitro explosives. In medicine, it is used as an additive in cream to rehydration of skin.
Health effects / safety hazards: Urea can cause irritation in eyes and respiratory system. It may also cause damage in the skin due its long term use. At high temperature, it can decomposes producing toxic vapors. Moreover, urea may react violently with some chemical compounds as nitrites and perchlorates.
|
Related Links: |
