Specific Gravity Formula
Specific gravity is a measure of relative density. The specific gravity is the density of a substance divided by the density of water. Density is measured in the units kg/m3. The density of water at 4.0°C is 1000 kg/m3. So, the specific gravity is a unitless number.

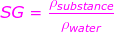
SG = specific gravity (unitless)
ρsubstance = the density of the substance (kg/m3)
ρwater = the density of water at 4.0°C, 1000 kg/m3
Specific Gravity Formula Questions:
1) The density of gold is ρgold = 19300 kg/m3. What is the specific gravity of gold?
Answer: The specific gravity can be found by dividing the density of gold by the density of water at 4.0°C:
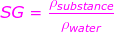
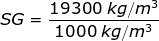
SG = 19.3
The specific gravity of gold is 19.3.
2) A The specific gravity of ice is 0.92. What is the density of ice, and is it greater or less than the density of water at 4.0°C (liquid water)?
Answer: The density of ice can be found by rearranging the specific gravity equation:
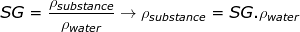
ρsubstance = 0.92∙1000 kg/m3
ρsubstance = 920 kg/m3
The density of ice is 920 kg/m3, which is less than the density of liquid water. This is a very rare property of water compared to other materials. The density of the solid form of a substance is almost always greater than the density of the liquid form. Besides water, the most "common" substance that has this property is plutonium.
|
Related Links: |
